当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
1,6-Conjugated addition-mediated [4 + 1] annulation: an approach to 2,3-dihydrobenzofurans
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1039/c7qo00846e Lina Liu 1, 2, 3, 4, 5 , Zhenbo Yuan 1, 2, 3, 4, 5 , Rui Pan 1, 2, 3, 4, 5 , Yuye Zeng 1, 2, 3, 4, 5 , Aijun Lin 1, 2, 3, 4, 5 , Hequan Yao 1, 2, 3, 4, 5 , Yue Huang 3, 4, 5, 6, 7
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1039/c7qo00846e Lina Liu 1, 2, 3, 4, 5 , Zhenbo Yuan 1, 2, 3, 4, 5 , Rui Pan 1, 2, 3, 4, 5 , Yuye Zeng 1, 2, 3, 4, 5 , Aijun Lin 1, 2, 3, 4, 5 , Hequan Yao 1, 2, 3, 4, 5 , Yue Huang 3, 4, 5, 6, 7
Affiliation
|
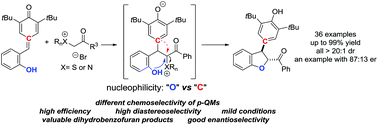
A formal 1,6-conjugated addition-mediated [4 + 1] annulation of ortho-hydroxyphenyl-substituted para-quinone methides with sulfonium or ammonium bromides has been described. This domino-type process offered an efficient method for the synthesis of 2,3-dihydrobenzofurans in good yields, exhibiting good functional group tolerance, scalability and high diastereoselectivity.
中文翻译:

1,6-共轭加成介导的[4 +1]环空:一种2,3-二氢苯并呋喃的方法
已经描述了正式的1,6-共轭加成介导的[4- + 1]邻-羟基苯基取代的对-醌甲基化物与sulf或溴化铵的环合。这种多米诺型工艺提供了一种高效,高收率地合成2,3-二氢苯并呋喃的方法,具有良好的官能团耐受性,可扩展性和高非对映选择性。
更新日期:2017-11-15
中文翻译:

1,6-共轭加成介导的[4 +1]环空:一种2,3-二氢苯并呋喃的方法
已经描述了正式的1,6-共轭加成介导的[4- + 1]邻-羟基苯基取代的对-醌甲基化物与sulf或溴化铵的环合。这种多米诺型工艺提供了一种高效,高收率地合成2,3-二氢苯并呋喃的方法,具有良好的官能团耐受性,可扩展性和高非对映选择性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号