当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enhanced Congo red dye removal from aqueous solutions using iron nanoparticles: adsorption, kinetics, and equilibrium studies
Dalton Transactions ( IF 4 ) Pub Date : 2017-11-01 00:00:00 , DOI: 10.1039/c7dt02076g Se-Ho Kim 1, 2, 3, 4 , Pyuck-Pa Choi 1, 2, 3, 4
Dalton Transactions ( IF 4 ) Pub Date : 2017-11-01 00:00:00 , DOI: 10.1039/c7dt02076g Se-Ho Kim 1, 2, 3, 4 , Pyuck-Pa Choi 1, 2, 3, 4
Affiliation
|
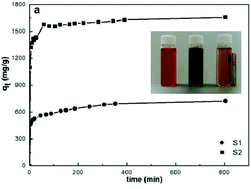
We report on the Congo red dye removal properties of body centred cubic and amorphous iron nanoparticles, synthesized by a facile borohydride reduction method under ambient conditions. We have analyzed the adsorption of Congo red as a function of dye concentration, time, and temperature and measured a Congo red adsorption capacity of 1735 mg g−1 for the amorphous iron nanoparticles. To our knowledge, this is the highest value reported so far for Congo red adsorption. The acquired data have been evaluated applying various models for adsorption kinetics and thermodynamic studies. The isotherm models as well as acquired Fourier transform infrared spectra suggest that both chemi- and physisorption occur for Congo red adsorption on iron nanoparticles, where chemisorption appears to be dominant. The kinetics of adsorption of Congo red on both bcc-structured and amorphous iron follow a pseudo-second order equation and are characterized by high initial adsorption rates. Diffusion studies indicate that adsorption occurs in two stages, namely film diffusion followed by intraparticle diffusion. Our studies show that amorphous iron nanoparticles are highly promising for dye adsorption and wastewater treatment applications.
中文翻译:

使用铁纳米颗粒增强水溶液中刚果红染料的去除:吸附,动力学和平衡研究
我们报告了在环境条件下通过简便的硼氢化物还原方法合成的体心立方和无定形铁纳米粒子的刚果红染料去除性能。我们分析了刚果红的吸附随染料浓度,时间和温度的变化,并测得刚果红的吸附容量为1735 mg g -1用于无定形铁纳米颗粒。据我们所知,这是迄今为止刚果红吸附报道的最高值。应用各种模型进行吸附动力学和热力学研究,对获得的数据进行了评估。等温线模型以及获得的傅立叶变换红外光谱表明,刚果红在铁纳米颗粒上的吸附同时发生了化学吸附和物理吸附,其中化学吸附似乎占主导地位。刚果红在bcc结构和非晶态铁上的吸附动力学均遵循伪二级方程,其特征是初始吸附速率高。扩散研究表明,吸附发生在两个阶段,即膜扩散和随后的颗粒内扩散。
更新日期:2017-11-15
中文翻译:

使用铁纳米颗粒增强水溶液中刚果红染料的去除:吸附,动力学和平衡研究
我们报告了在环境条件下通过简便的硼氢化物还原方法合成的体心立方和无定形铁纳米粒子的刚果红染料去除性能。我们分析了刚果红的吸附随染料浓度,时间和温度的变化,并测得刚果红的吸附容量为1735 mg g -1用于无定形铁纳米颗粒。据我们所知,这是迄今为止刚果红吸附报道的最高值。应用各种模型进行吸附动力学和热力学研究,对获得的数据进行了评估。等温线模型以及获得的傅立叶变换红外光谱表明,刚果红在铁纳米颗粒上的吸附同时发生了化学吸附和物理吸附,其中化学吸附似乎占主导地位。刚果红在bcc结构和非晶态铁上的吸附动力学均遵循伪二级方程,其特征是初始吸附速率高。扩散研究表明,吸附发生在两个阶段,即膜扩散和随后的颗粒内扩散。


























 京公网安备 11010802027423号
京公网安备 11010802027423号