当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Understanding the Regioselectivity of Aromatic Hydroxylation over Divanadium-Substituted γ-Keggin Polyoxotungstate
ACS Catalysis ( IF 12.9 ) Pub Date : 2017-11-15 00:00:00 , DOI: 10.1021/acscatal.7b02694 Igor Y. Skobelev 1, 2 , Vasiliy Yu. Evtushok 1, 2 , Oxana A. Kholdeeva 1, 2 , Nataliya V. Maksimchuk 1, 2 , Raisa I. Maksimovskaya 1 , Josep M. Ricart 3 , Josep M. Poblet 3 , Jorge J. Carbó 3
ACS Catalysis ( IF 12.9 ) Pub Date : 2017-11-15 00:00:00 , DOI: 10.1021/acscatal.7b02694 Igor Y. Skobelev 1, 2 , Vasiliy Yu. Evtushok 1, 2 , Oxana A. Kholdeeva 1, 2 , Nataliya V. Maksimchuk 1, 2 , Raisa I. Maksimovskaya 1 , Josep M. Ricart 3 , Josep M. Poblet 3 , Jorge J. Carbó 3
Affiliation
|
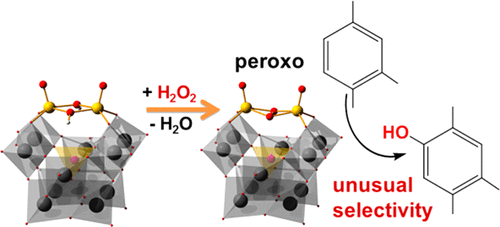
The aromatic hydroxylation of pseudocumene (PC) with aqueous hydrogen peroxide catalyzed by the divanadium-substituted γ-Keggin polyoxotungstate TBA4[γ-PW10O38V2(μ-O)(μ-OH)] (TBA-1H, TBA = tetrabutylammonium) has been studied using kinetic modeling and DFT calculations. This reaction features high chemoselectivity and unusual regioselectivity, affording 2,4,5-trimethylphenol (TMP) as the main product. Then the computational study was extended to the analysis of the regioselectivity for other alkoxy- and alkylarene substrates. The protonation/deprotonation of TBA-1H in MeCN/tBuOH (1:1) was investigated by 31P NMR spectroscopy. Forms with different protonation states, [γ-PV2W10O40]5– (1), [γ-HPV2W10O40]4– (1H), and [γ-H2PV2W10O40]3– (1H2), have been identified, and the protonation equilibrium constants were estimated on the basis of the 31P NMR data. DFT calculations were used to investigate the oxygen transfer process from hydroperoxo species, [γ-PW10O38V2(μ-O)(μ-OOH)]4– (2) and [γ-PW10O38V2(μ-OH)(μ-OOH)]3– (2H), and peroxo complex [γ-PW10O38V2(μ-η2:η2-O2)]3– (3) toward the different positions in the aromatic ring of PC, anisole, and toluene substrates. Product, kinetic, and computational studies on the PC hydroxylation strongly support a mechanism of electrophilic oxygen atom transfer from peroxo complex 3 to the aromatic ring of PC. The kinetic modeling revealed that the contribution of 3 into the initial reaction rate is, on average, about 70%, but it may depend on the reaction conditions. DFT calculations showed that the steric hindrance exerted by peroxo complex 3 is responsible for the origin of the unusual regioselectivity observed in PC hydroxylation, while for anisole and toluene the regioselective para-hydroxylation is due to electronic preference during the oxygen transfer from the active peroxo species 3.
中文翻译:

理解芳烃羟基取代在钒取代的γ-Keggin聚氧钨酸盐上的区域选择性
假枯(PC)用含水氢的芳香族羟基化过氧化物由二钒取代的γ-的Keggin polyoxotungstate TBA催化的4 [γ-PW 10 ö 38 V 2(μ-O)(μ-OH)](TBA- 1H,TBA =四丁基铵)已使用动力学建模和DFT计算进行了研究。该反应具有很高的化学选择性和异常的区域选择性,提供了2,4,5-三甲基苯酚(TMP)作为主要产物。然后将计算研究扩展到对其他烷氧基和烷基芳烃底物的区域选择性的分析。用31研究了MeCN / t BuOH(1:1)中TBA- 1H的质子化/去质子化1 H NMR光谱。用不同的质子化状态,[γ-PV形式2 w ^ 10 ö 40 ] 5-(1),[γ-HPV 2 w ^ 10 ö 40 ] 4-(1H),和[γ-H 2 PV 2 W ^ 10 Ô 40 ] 3–(1H 2),已经确定,并且质子平衡常数是根据31 P NMR数据估算的。DFT计算被用来研究从氢过物种的氧转移过程中,[γ-PW 10 ö 38V 2(μ-O)(μ-OOH)] 4-(2)和[γ-PW 10 ö 38 V 2(μ-OH)(μ-OOH)] 3-(2H),和过氧络合物[γ -PW 10 ø 38 V 2(μ-η 2:η 2 -O 2)] 3-(3)朝向PC,苯甲醚,甲苯和底物的芳环中的不同位置。PC羟基化的产物,动力学和计算研究强烈支持过氧化物络合物3亲电性氧原子转移的机理到PC的芳环。动力学模型表明,3对初始反应速率的贡献平均约为70%,但这可能取决于反应条件。DFT计算表明,过氧配合物3施加的空间位阻是PC羟基化过程中观察到的异常区域选择性的起因,而苯甲醚和甲苯的区域选择性对羟基化是由于从活性过氧物质转移氧过程中的电子偏好而引起的。3。
更新日期:2017-11-15
中文翻译:

理解芳烃羟基取代在钒取代的γ-Keggin聚氧钨酸盐上的区域选择性
假枯(PC)用含水氢的芳香族羟基化过氧化物由二钒取代的γ-的Keggin polyoxotungstate TBA催化的4 [γ-PW 10 ö 38 V 2(μ-O)(μ-OH)](TBA- 1H,TBA =四丁基铵)已使用动力学建模和DFT计算进行了研究。该反应具有很高的化学选择性和异常的区域选择性,提供了2,4,5-三甲基苯酚(TMP)作为主要产物。然后将计算研究扩展到对其他烷氧基和烷基芳烃底物的区域选择性的分析。用31研究了MeCN / t BuOH(1:1)中TBA- 1H的质子化/去质子化1 H NMR光谱。用不同的质子化状态,[γ-PV形式2 w ^ 10 ö 40 ] 5-(1),[γ-HPV 2 w ^ 10 ö 40 ] 4-(1H),和[γ-H 2 PV 2 W ^ 10 Ô 40 ] 3–(1H 2),已经确定,并且质子平衡常数是根据31 P NMR数据估算的。DFT计算被用来研究从氢过物种的氧转移过程中,[γ-PW 10 ö 38V 2(μ-O)(μ-OOH)] 4-(2)和[γ-PW 10 ö 38 V 2(μ-OH)(μ-OOH)] 3-(2H),和过氧络合物[γ -PW 10 ø 38 V 2(μ-η 2:η 2 -O 2)] 3-(3)朝向PC,苯甲醚,甲苯和底物的芳环中的不同位置。PC羟基化的产物,动力学和计算研究强烈支持过氧化物络合物3亲电性氧原子转移的机理到PC的芳环。动力学模型表明,3对初始反应速率的贡献平均约为70%,但这可能取决于反应条件。DFT计算表明,过氧配合物3施加的空间位阻是PC羟基化过程中观察到的异常区域选择性的起因,而苯甲醚和甲苯的区域选择性对羟基化是由于从活性过氧物质转移氧过程中的电子偏好而引起的。3。



























 京公网安备 11010802027423号
京公网安备 11010802027423号