当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrochemical Reduction of CO2 on IrxRu(1–x)O2(110) Surfaces
ACS Catalysis ( IF 12.9 ) Pub Date : 2017-11-15 00:00:00 , DOI: 10.1021/acscatal.7b02914 Arghya Bhowmik 1 , Heine Anton Hansen 1 , Tejs Vegge 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2017-11-15 00:00:00 , DOI: 10.1021/acscatal.7b02914 Arghya Bhowmik 1 , Heine Anton Hansen 1 , Tejs Vegge 1
Affiliation
|
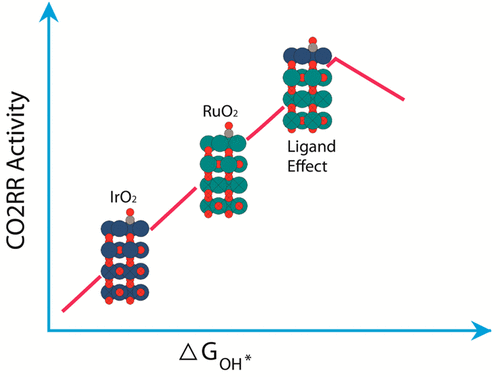
High overpotentials and low faradic efficiencies plague metal catalysts for direct conversion of CO2 to methanol and other liquid fuels. RuO2-based electrocatalysts have been observed to evolve methanol at low overpotentials, which has been attributed to an alternative reaction mechanism with oxygen-coordinated intermediates that can circumvent the limitations imposed by the scaling relations on metal catalysts. Here, we introduce an innovative concept of ligand effects in oxide catalysts. Both IrO2 and RuO2 binds OH* and other intermediates from the electrochemical reduction of CO2 (CO2RR) strongly, but the stable and miscible system IrxRu(1-x)O2 exhibits anomalous weaker binding energy in the presence of CO* spectators, because of Ru–Ir ligand effects. The weakened adsorbate binding leads to a very low CO2RR onset potential (methanol evolution at −0.2 V RHE). An Ir atom at the bridge site with Ru neighbors binds intermediates such as OH* and OCHO* much weaker, because of synergistic ligand effects and adsorbate–adsorbate interactions. Consequently, a RuO2 surface doped with Ir move close to the top of the predicted CO2RR volcano for oxides, which offers a significant improvement over state-of-the-art electrocatalysts for conversion of CO2 into methanol. Analysis of electronic structure parameters with adsorbate binding energies indicates the ligand effect depletes electrons from the Ir atom and shifts the t2g orbitals. The lack of electron donation from CO* spectators to Ir at the active site cause favorable adsorbate binding.
中文翻译:

Ir x Ru (1– x) O 2(110)表面上的CO 2电化学还原
高过电势和低法拉第效率困扰着金属催化剂,这些催化剂将CO 2直接转化为甲醇和其他液体燃料。已经观察到基于RuO 2的电催化剂在低的超电势下释放出甲醇,这归因于具有氧配位中间体的替代反应机理,该机理可以规避金属催化剂上结垢关系所施加的限制。在这里,我们介绍了氧化物催化剂中配体效应的创新概念。IrO 2和RuO 2都牢固地结合OH *和来自电化学还原CO 2(CO2RR)的其他中间体,但是稳定且可混溶的系统Ir x Ru (1-x) O2由于Ru–Ir配体效应,在CO *旁观者的存在下表现出异常弱的结合能。减弱的吸附物结合导致非常低的CO2RR起始电位(甲醇在-0.2 V RHE处放出)。由于与配体的协同作用和吸附物-吸附物的相互作用,与Ru相邻的桥位处的Ir原子与OH *和OCHO *等中间体的结合要弱得多。因此,掺有Ir的RuO 2表面接近氧化物预测的CO2RR火山的顶部,这相对于将CO 2转化为甲醇的最先进的电催化剂而言,具有显着的改进。具有吸附质结合能的电子结构参数分析表明,配体效应耗尽了Ir原子中的电子并移动了t2g轨道。在活性位点,CO *观众缺乏向Ir的电子给体,导致了良好的吸附物结合。
更新日期:2017-11-15
中文翻译:

Ir x Ru (1– x) O 2(110)表面上的CO 2电化学还原
高过电势和低法拉第效率困扰着金属催化剂,这些催化剂将CO 2直接转化为甲醇和其他液体燃料。已经观察到基于RuO 2的电催化剂在低的超电势下释放出甲醇,这归因于具有氧配位中间体的替代反应机理,该机理可以规避金属催化剂上结垢关系所施加的限制。在这里,我们介绍了氧化物催化剂中配体效应的创新概念。IrO 2和RuO 2都牢固地结合OH *和来自电化学还原CO 2(CO2RR)的其他中间体,但是稳定且可混溶的系统Ir x Ru (1-x) O2由于Ru–Ir配体效应,在CO *旁观者的存在下表现出异常弱的结合能。减弱的吸附物结合导致非常低的CO2RR起始电位(甲醇在-0.2 V RHE处放出)。由于与配体的协同作用和吸附物-吸附物的相互作用,与Ru相邻的桥位处的Ir原子与OH *和OCHO *等中间体的结合要弱得多。因此,掺有Ir的RuO 2表面接近氧化物预测的CO2RR火山的顶部,这相对于将CO 2转化为甲醇的最先进的电催化剂而言,具有显着的改进。具有吸附质结合能的电子结构参数分析表明,配体效应耗尽了Ir原子中的电子并移动了t2g轨道。在活性位点,CO *观众缺乏向Ir的电子给体,导致了良好的吸附物结合。


























 京公网安备 11010802027423号
京公网安备 11010802027423号