Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Dependence of the Electrochemical Redox Properties of Fullerenes on Ionic Liquids
Langmuir ( IF 3.9 ) Pub Date : 2017-11-14 00:00:00 , DOI: 10.1021/acs.langmuir.7b03076 Hiroyuki Ueda , Tomoaki Yoshimura , Katsuhiko Nishiyama 1 , Soichiro Yoshimoto 1
Langmuir ( IF 3.9 ) Pub Date : 2017-11-14 00:00:00 , DOI: 10.1021/acs.langmuir.7b03076 Hiroyuki Ueda , Tomoaki Yoshimura , Katsuhiko Nishiyama 1 , Soichiro Yoshimoto 1
Affiliation
|
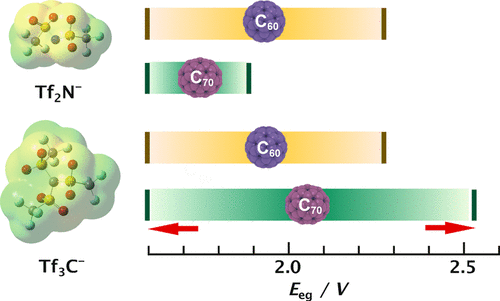
Control of the electrochemical properties of fullerenes via the chemical modification approach has attracted considerable attention. However, surface modification of fullerene cages with various functional groups can lead to the destruction of their original structures. Herein, we report a simple approach for controlling the electrochemical properties of fullerene thin films formed on Au(111) electrodes in various ionic liquids (ILs). A total of eight reversible redox couples for six reductive and two oxidative processes were observed for fullerenes in ammonium- and pyrrolidinium-based ILs. The redox potentials, the differences between two successive redox potentials, the average values of these potential differences for fullerene reduction, and the electrochemical band gaps of fullerene films in various ILs were found to depend on the cation and its alkyl chain length, the anion, and the chemical structure of the fullerene. Highly charged C70 anions were reduced more easily than C60 anions. An increase in the alkyl chain length of the cation led to an increase in the average potential difference between two successive redox potentials for fullerene reduction. The results indicate that the electrochemical band gaps of fullerenes can be manipulated using ILs with appropriate anions, which can be determined based on the size of the anion and the charge distribution.
中文翻译:

富勒烯的电化学氧化还原性质对离子液体的依赖性
通过化学改性方法控制富勒烯的电化学性质已引起相当大的关注。然而,具有各种官能团的富勒烯笼的表面改性可导致其原始结构的破坏。在这里,我们报告了一种简单的方法,用于控制在各种离子液体(IL)中在Au(111)电极上形成的富勒烯薄膜的电化学性能。在基于铵和吡咯烷鎓的离子液体中,对于富勒烯共观察到八种可逆的氧化还原对,分别用于六个还原和两个氧化过程。氧化还原电势,两个连续氧化还原电势之间的差,富勒烯还原时这些电势差的平均值,富勒烯薄膜在不同离子液体中的电化学带隙取决于阳离子及其烷基链长,阴离子和富勒烯的化学结构。高电荷C70种阴离子比60种阴离子更容易还原。阳离子烷基链长度的增加导致富勒烯还原的两个连续氧化还原电势之间的平均电势差增加。结果表明,富勒烯的电化学带隙可以使用带有适当阴离子的IL来控制,这可以基于阴离子的大小和电荷分布来确定。
更新日期:2017-11-14
中文翻译:

富勒烯的电化学氧化还原性质对离子液体的依赖性
通过化学改性方法控制富勒烯的电化学性质已引起相当大的关注。然而,具有各种官能团的富勒烯笼的表面改性可导致其原始结构的破坏。在这里,我们报告了一种简单的方法,用于控制在各种离子液体(IL)中在Au(111)电极上形成的富勒烯薄膜的电化学性能。在基于铵和吡咯烷鎓的离子液体中,对于富勒烯共观察到八种可逆的氧化还原对,分别用于六个还原和两个氧化过程。氧化还原电势,两个连续氧化还原电势之间的差,富勒烯还原时这些电势差的平均值,富勒烯薄膜在不同离子液体中的电化学带隙取决于阳离子及其烷基链长,阴离子和富勒烯的化学结构。高电荷C70种阴离子比60种阴离子更容易还原。阳离子烷基链长度的增加导致富勒烯还原的两个连续氧化还原电势之间的平均电势差增加。结果表明,富勒烯的电化学带隙可以使用带有适当阴离子的IL来控制,这可以基于阴离子的大小和电荷分布来确定。


























 京公网安备 11010802027423号
京公网安备 11010802027423号