当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The effect of CeO2–ZrO2 structural differences on the origin and reactivity of carbon formed during methane dry reforming over NiCo/CeO2–ZrO2 catalysts studied by transient techniques
Catalysis Science & Technology ( IF 5 ) Pub Date : 2017-08-17 00:00:00 , DOI: 10.1039/c7cy01009e Michalis A. Vasiliades 1, 2, 3, 4, 5 , Petar Djinović 6, 7, 8, 9 , Albin Pintar 6, 7, 8, 9 , Janez Kovač 8, 9, 10, 11 , Angelos M. Efstathiou 1, 2, 3, 4, 5
Catalysis Science & Technology ( IF 5 ) Pub Date : 2017-08-17 00:00:00 , DOI: 10.1039/c7cy01009e Michalis A. Vasiliades 1, 2, 3, 4, 5 , Petar Djinović 6, 7, 8, 9 , Albin Pintar 6, 7, 8, 9 , Janez Kovač 8, 9, 10, 11 , Angelos M. Efstathiou 1, 2, 3, 4, 5
Affiliation
|
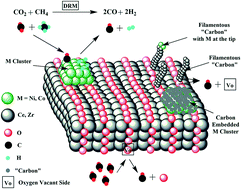
Nickel (1.2 wt%) and cobalt (1.8 wt%) were dispersed over Ce0.75Zr0.25O2−δ solid solution (3NiCo EG) or over a mixture of CeO2 and ZrO2 single phases (3NiCo HT) and tested for dry reforming of methane (DRM) at 750 °C. The structural, morphological, textural and redox differences between 3NiCo EG and 3NiCo HT catalysts were probed by powder XRD, HAADF/STEM and SAED, N2 adsorption/desorption at 77 K, H2-chemisorption, H2-TPR and H2 transient isothermal reduction (H2-TIR) techniques. The origin, concentration and reactivity of “carbon” deposits formed in DRM (via the CH4 and CO2 activation routes) towards gaseous H2 and O2 but also towards the support's labile oxygen species in the prepared catalysts were analyzed by a series of various transient and SSITKA experiments (use of 18O2 and 13CO2). Regardless of the EG or HT support, the %-contribution of CH4 and CO2 to the “carbon” deposition is very similar but the amount and reactivity towards oxidation are largely different. On the other hand, the concentration of active carbon formed in the carbon pathway of the CO2 activation route is very small (θC < 0.16% after 2 h in DRM). Participation of mobile lattice oxygen species in gasification of deposited “carbon” to CO(g) does occur to a large extent on both EG- and HT-supported NiCo catalysts. The catalyst deactivation rate during the first 5 h of DRM was found to depend on the structure of the support (EG vs. HT). The faster deactivation observed with the 3NiCo EG catalyst cannot be linked to the existing differences in the rates of inactive “carbon” formation and depletion or the concentration of active carbon but rather to the different rates of encapsulation of NiCo bimetallic particles in the carbon layers formed (∼30 nm thick) as revealed by HAADF/STEM.
中文翻译:

瞬态技术研究NiO / CeO 2 -ZrO 2催化剂上甲烷干重整过程中CeO 2 -ZrO 2结构差异对碳源和反应活性的影响
镍(1.2重量%)和钴(1.8重量%)分散在铈0.75锆0.25 ö 2- δ固溶体(3NiCo EG)或以上的CeO的混合物2和ZrO 2个的单相(3NiCo HT),并测试干燥750°C下的甲烷(DRM)重整。3NiCo EG和3NiCo HT催化剂之间的结构,形态,质地和氧化还原差异通过粉末XRD进行探测,HAADF / STEM和SAED,N 2在77K的吸附/解吸,H 2 -chemisorption,H 2 -TPR和H 2瞬变等温还原(H 2 -TIR)技术。DRM中形成的“碳”沉积物的来源,浓度和反应性(通过CH 4和CO 2活化途径)向气态H 2和O 2以及制备的催化剂中对载体的不稳定氧物种进行了分析,方法是通过一系列各种瞬态和SSITKA实验(使用18 O 2和13 CO 2)。不管是EG还是HT载体,CH 4和CO 2对“碳”沉积的%贡献都非常相似,但对氧化的量和反应性却大不相同。另一方面,在CO 2的碳路径中形成的活性炭的浓度活化途径是非常小(θ ç <0.16%在DRM 2小时后)。在EG和HT负载的NiCo催化剂上,都确实发生了可移动晶格氧物种在很大程度上将沉积的“碳”气化为CO(g)的现象。发现在DRM的前5小时内催化剂失活速率取决于载体的结构(EG对HT)。用3NiCo EG催化剂观察到的更快的失活不能与无活性“碳”形成和耗尽的速率或活性炭浓度存在的差异有关,而与所形成的碳层中NiCo双金属颗粒的包封速率不同有关(〜30 nm厚),如HAADF / STEM所揭示。
更新日期:2017-11-14
中文翻译:

瞬态技术研究NiO / CeO 2 -ZrO 2催化剂上甲烷干重整过程中CeO 2 -ZrO 2结构差异对碳源和反应活性的影响
镍(1.2重量%)和钴(1.8重量%)分散在铈0.75锆0.25 ö 2- δ固溶体(3NiCo EG)或以上的CeO的混合物2和ZrO 2个的单相(3NiCo HT),并测试干燥750°C下的甲烷(DRM)重整。3NiCo EG和3NiCo HT催化剂之间的结构,形态,质地和氧化还原差异通过粉末XRD进行探测,HAADF / STEM和SAED,N 2在77K的吸附/解吸,H 2 -chemisorption,H 2 -TPR和H 2瞬变等温还原(H 2 -TIR)技术。DRM中形成的“碳”沉积物的来源,浓度和反应性(通过CH 4和CO 2活化途径)向气态H 2和O 2以及制备的催化剂中对载体的不稳定氧物种进行了分析,方法是通过一系列各种瞬态和SSITKA实验(使用18 O 2和13 CO 2)。不管是EG还是HT载体,CH 4和CO 2对“碳”沉积的%贡献都非常相似,但对氧化的量和反应性却大不相同。另一方面,在CO 2的碳路径中形成的活性炭的浓度活化途径是非常小(θ ç <0.16%在DRM 2小时后)。在EG和HT负载的NiCo催化剂上,都确实发生了可移动晶格氧物种在很大程度上将沉积的“碳”气化为CO(g)的现象。发现在DRM的前5小时内催化剂失活速率取决于载体的结构(EG对HT)。用3NiCo EG催化剂观察到的更快的失活不能与无活性“碳”形成和耗尽的速率或活性炭浓度存在的差异有关,而与所形成的碳层中NiCo双金属颗粒的包封速率不同有关(〜30 nm厚),如HAADF / STEM所揭示。



























 京公网安备 11010802027423号
京公网安备 11010802027423号