当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Kinetics of hydrogen adsorption during catalytic reactions on transition metal surfaces
Catalysis Science & Technology ( IF 5 ) Pub Date : 2017-07-06 00:00:00 , DOI: 10.1039/c7cy00216e Yujung Dong 1, 2, 3, 4 , Francisco Zaera 1, 2, 3, 4
Catalysis Science & Technology ( IF 5 ) Pub Date : 2017-07-06 00:00:00 , DOI: 10.1039/c7cy00216e Yujung Dong 1, 2, 3, 4 , Francisco Zaera 1, 2, 3, 4
Affiliation
|
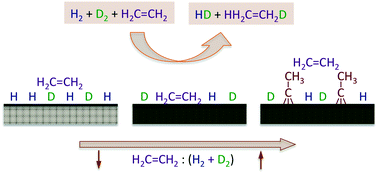
A study of the kinetics of the hydrogenation of ethylene promoted by hydrogen was performed by using a high-flux molecular beam. The experiments were designed to probe the intermediate pressure regime (around the mTorr range) associated with the transition between the surface-science research carried out under ultrahigh vacuum (UHV) environments and the catalytic studies performed under atmospheric conditions, the so-called “pressure gap” that is often mentioned but seldom addressed in catalytic work. In addition, the experiments were also focused on the characterization of the kinetics of both hydrogen isotope scrambling and ethylene hydrogenation simultaneously, by using H2 + D2 + C2H4 reaction mixtures and by following the time evolution of all of the products, HD and the different C2X6 isotopologues (X = H or D), in order to estimate the influence of the kinetics of the hydrogen uptake on the overall olefin conversion rates under reaction conditions. It was found that the addition of ethylene to the H2 + D2 beam leads to a significant decrease in the probability for HD production, but that the olefin hydrogenation reaction can still be sustained catalytically under the conditions of our experiments. Three kinetic regimes were identified with increasing partial pressure (or flux) of ethylene in the reaction mixture. The first, seen for mixtures with less than 10 parts per million of ethylene, shows a steady-state production of ethane with kinetics similar to those reported from UHV studies, with a rate law dependent linearly on both ethylene partial pressure and hydrogen atom coverage. The surface is mainly covered with hydrogen, and ethane formation occurs primarily via a previously unidentified “reverse” Eley–Rideal mechanism where olefin molecules from the gas phase pick up two hydrogen (deuterium) atoms upon impingement on the surface. A second, intermediate regime is seen for mixtures with up to about 1% of ethylene. In that case, HD production is still relatively fast, albeit the rate decreases slowly with increasing ethylene pressure, and the catalytic activity is mainly controlled by the coverage of the reversibly adsorbed ethylene, which partially blocks the hydrogen uptake (some di-σ ethylene and ethylidyne, Pt3![[triple bond, length as m-dash]](http://www.rsc.org/images/entities/char_e002.gif) C–CH3, a species that forms via ethylene dehydrogenation, are also strongly adsorbed on the surface). The probability for ethane formation decreases noticeably in this regime, and the reaction mechanism switches to the stepwise hydrogen incorporation sequence proposed by Horiuti and Polanyi many years ago. Finally, for reaction mixtures with more than 1% of ethylene, the ethylidyne surface layer reaches coverages close to saturation and controls the HD and ethane formation kinetics via site blocking; this latter regime is the one operational under most typical catalytic runs. It was also shown that the relative importance of the reversibly adsorbed ethylene and irreversibly adsorbed ethylidyne species in the reaction kinetics depends on surface temperature, and that the ethylidyne layer can be removed at temperatures around 500 K to restore the full catalytic activity of the clean Pt surface.
C–CH3, a species that forms via ethylene dehydrogenation, are also strongly adsorbed on the surface). The probability for ethane formation decreases noticeably in this regime, and the reaction mechanism switches to the stepwise hydrogen incorporation sequence proposed by Horiuti and Polanyi many years ago. Finally, for reaction mixtures with more than 1% of ethylene, the ethylidyne surface layer reaches coverages close to saturation and controls the HD and ethane formation kinetics via site blocking; this latter regime is the one operational under most typical catalytic runs. It was also shown that the relative importance of the reversibly adsorbed ethylene and irreversibly adsorbed ethylidyne species in the reaction kinetics depends on surface temperature, and that the ethylidyne layer can be removed at temperatures around 500 K to restore the full catalytic activity of the clean Pt surface.
中文翻译:

过渡金属表面催化反应过程中氢吸附的动力学
使用高通量分子束对氢促进乙烯加氢的动力学进行了研究。设计这些实验是为了探索与在超高真空(UHV)环境下进行的表面科学研究与在大气条件下进行的催化研究之间的过渡相关的中间压力范围(在mTorr范围内)。差距”,但在催化工作中却很少提及。此外,实验还着重于通过使用H 2 + D 2 + C 2 H 4同时表征氢同位素加扰和乙烯加氢的动力学。反应混合物,并遵循所有产物,HD和不同的C 2 X 6同位素异构体(X = H或D)的时间演化,以估计氢吸收动力学对总烯烃转化率的影响在反应条件下。发现向H 2 + D 2中添加乙烯束导致产生HD的可能性显着降低,但是在我们的实验条件下,烯烃氢化反应仍可以催化持续进行。随着反应混合物中乙烯分压(或通量)的增加,确定了三种动力学方案。对于乙烯含量低于百万分之十的混合物,第一个反应物显示出乙烷的稳态生产,其动力学与超高压研究报告的动力学相似,速率规律线性地取决于乙烯分压和氢原子覆盖率。表面主要被氢覆盖,乙烷的形成主要通过以前未知的“反向” Eley-Rideal机理,其中气相中的烯烃分子撞击表面时会吸收两个氢(氘)原子。对于具有最多约1%的乙烯的混合物,可以看到第二种中间方案。在那种情况下,HD的生产仍然相对较快,尽管其速率随着乙烯压力的增加而缓慢降低,并且催化活性主要受可逆吸附乙烯的覆盖率控制,这部分阻止了氢的吸收(某些di-σ乙烯和乙炔,Pt 3![[三重键,长度为m-破折号]](http://www.rsc.org/images/entities/char_e002.gif) C–CH 3,是通过乙烯脱氢,也很强地吸附在表面上)。在这种情况下,乙烷形成的可能性显着降低,反应机理切换到Horiuti和Polanyi提出的逐步引入氢的序列。最后,用于与乙烯的1%以上的反应混合物中,乙川表面层达到覆盖率接近饱和和控制HD和乙烷的形成动力学经由网站封锁;后一种方式是在最典型的催化运行下可操作的一种方式。还表明,可逆吸附的乙烯和不可逆吸附的乙炔物种在反应动力学中的相对重要性取决于表面温度,并且乙炔层可以在约500 K的温度下去除,以恢复纯净Pt的全部催化活性。表面。
C–CH 3,是通过乙烯脱氢,也很强地吸附在表面上)。在这种情况下,乙烷形成的可能性显着降低,反应机理切换到Horiuti和Polanyi提出的逐步引入氢的序列。最后,用于与乙烯的1%以上的反应混合物中,乙川表面层达到覆盖率接近饱和和控制HD和乙烷的形成动力学经由网站封锁;后一种方式是在最典型的催化运行下可操作的一种方式。还表明,可逆吸附的乙烯和不可逆吸附的乙炔物种在反应动力学中的相对重要性取决于表面温度,并且乙炔层可以在约500 K的温度下去除,以恢复纯净Pt的全部催化活性。表面。
更新日期:2017-11-14
![[triple bond, length as m-dash]](http://www.rsc.org/images/entities/char_e002.gif) C–CH3, a species that forms via ethylene dehydrogenation, are also strongly adsorbed on the surface). The probability for ethane formation decreases noticeably in this regime, and the reaction mechanism switches to the stepwise hydrogen incorporation sequence proposed by Horiuti and Polanyi many years ago. Finally, for reaction mixtures with more than 1% of ethylene, the ethylidyne surface layer reaches coverages close to saturation and controls the HD and ethane formation kinetics via site blocking; this latter regime is the one operational under most typical catalytic runs. It was also shown that the relative importance of the reversibly adsorbed ethylene and irreversibly adsorbed ethylidyne species in the reaction kinetics depends on surface temperature, and that the ethylidyne layer can be removed at temperatures around 500 K to restore the full catalytic activity of the clean Pt surface.
C–CH3, a species that forms via ethylene dehydrogenation, are also strongly adsorbed on the surface). The probability for ethane formation decreases noticeably in this regime, and the reaction mechanism switches to the stepwise hydrogen incorporation sequence proposed by Horiuti and Polanyi many years ago. Finally, for reaction mixtures with more than 1% of ethylene, the ethylidyne surface layer reaches coverages close to saturation and controls the HD and ethane formation kinetics via site blocking; this latter regime is the one operational under most typical catalytic runs. It was also shown that the relative importance of the reversibly adsorbed ethylene and irreversibly adsorbed ethylidyne species in the reaction kinetics depends on surface temperature, and that the ethylidyne layer can be removed at temperatures around 500 K to restore the full catalytic activity of the clean Pt surface.
中文翻译:

过渡金属表面催化反应过程中氢吸附的动力学
使用高通量分子束对氢促进乙烯加氢的动力学进行了研究。设计这些实验是为了探索与在超高真空(UHV)环境下进行的表面科学研究与在大气条件下进行的催化研究之间的过渡相关的中间压力范围(在mTorr范围内)。差距”,但在催化工作中却很少提及。此外,实验还着重于通过使用H 2 + D 2 + C 2 H 4同时表征氢同位素加扰和乙烯加氢的动力学。反应混合物,并遵循所有产物,HD和不同的C 2 X 6同位素异构体(X = H或D)的时间演化,以估计氢吸收动力学对总烯烃转化率的影响在反应条件下。发现向H 2 + D 2中添加乙烯束导致产生HD的可能性显着降低,但是在我们的实验条件下,烯烃氢化反应仍可以催化持续进行。随着反应混合物中乙烯分压(或通量)的增加,确定了三种动力学方案。对于乙烯含量低于百万分之十的混合物,第一个反应物显示出乙烷的稳态生产,其动力学与超高压研究报告的动力学相似,速率规律线性地取决于乙烯分压和氢原子覆盖率。表面主要被氢覆盖,乙烷的形成主要通过以前未知的“反向” Eley-Rideal机理,其中气相中的烯烃分子撞击表面时会吸收两个氢(氘)原子。对于具有最多约1%的乙烯的混合物,可以看到第二种中间方案。在那种情况下,HD的生产仍然相对较快,尽管其速率随着乙烯压力的增加而缓慢降低,并且催化活性主要受可逆吸附乙烯的覆盖率控制,这部分阻止了氢的吸收(某些di-σ乙烯和乙炔,Pt 3
![[三重键,长度为m-破折号]](http://www.rsc.org/images/entities/char_e002.gif) C–CH 3,是通过乙烯脱氢,也很强地吸附在表面上)。在这种情况下,乙烷形成的可能性显着降低,反应机理切换到Horiuti和Polanyi提出的逐步引入氢的序列。最后,用于与乙烯的1%以上的反应混合物中,乙川表面层达到覆盖率接近饱和和控制HD和乙烷的形成动力学经由网站封锁;后一种方式是在最典型的催化运行下可操作的一种方式。还表明,可逆吸附的乙烯和不可逆吸附的乙炔物种在反应动力学中的相对重要性取决于表面温度,并且乙炔层可以在约500 K的温度下去除,以恢复纯净Pt的全部催化活性。表面。
C–CH 3,是通过乙烯脱氢,也很强地吸附在表面上)。在这种情况下,乙烷形成的可能性显着降低,反应机理切换到Horiuti和Polanyi提出的逐步引入氢的序列。最后,用于与乙烯的1%以上的反应混合物中,乙川表面层达到覆盖率接近饱和和控制HD和乙烷的形成动力学经由网站封锁;后一种方式是在最典型的催化运行下可操作的一种方式。还表明,可逆吸附的乙烯和不可逆吸附的乙炔物种在反应动力学中的相对重要性取决于表面温度,并且乙炔层可以在约500 K的温度下去除,以恢复纯净Pt的全部催化活性。表面。


























 京公网安备 11010802027423号
京公网安备 11010802027423号