当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanistic study of the role of Au, Pd and Au–Pd in the surface reactions of ethanol over TiO2 in the dark and under photo-excitation
Catalysis Science & Technology ( IF 5 ) Pub Date : 2017-06-27 00:00:00 , DOI: 10.1039/c7cy00961e Shahid Bashir 1, 2, 3 , Hicham Idriss 1, 2, 3
Catalysis Science & Technology ( IF 5 ) Pub Date : 2017-06-27 00:00:00 , DOI: 10.1039/c7cy00961e Shahid Bashir 1, 2, 3 , Hicham Idriss 1, 2, 3
Affiliation
|
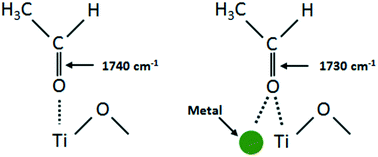
In situ infrared spectroscopy (FTIR) and catalytic reactions are employed to explore the photo-oxidation and photo-reforming of ethanol over TiO2 and M/TiO2 (M = Au, Pd and Au–Pd) catalysts. The effect of metal loadings (in mono- and bimetallic systems), the presence and absence of gas phase oxygen and the role of water are investigated. TEM images of the Au–Pd/TiO2 sample indicate that the mean Au particle size is 3.6 nm while that of Pd is 1.4 nm. Reaction intermediates on the surface during photo-oxidation of ethanol include carbon monoxide, acetaldehyde, acetate, formate and carbonate/bicarbonate species. The extent of formation and stability of adsorbed acetaldehyde, generated by dehydrogenation of ethanol, are in the order Au–Pd/TiO2 > Pd/TiO2 > Au/TiO2 > TiO2. Carboxylates predominantly appeared on all catalysts, while carbon monoxide (under inert flow) and carbonates/bicarbonates (under an O2 environment) are specifically produced on catalysts containing higher loading of Pd in both mono- and bimetallic systems. During photo-catalytic hydrogen production in the presence of ethanol/water at a light flux of 4–5 mW cm−2 (excitation energy = 3.44 eV), a maximum hydrogen production rate of 2.0 × 10−4 mol min−1 gcatalyst−1 is obtained with the Au–Pd/TiO2 catalyst (higher than those on its monometallic counterparts). The presence of Au in the bimetallic catalysts kept Pd in its metallic state as evidenced from UV-vis and X-ray photoelectron spectroscopy studies due to the enhanced carrier dynamics, facilitating charge transfer from Au to Pd, generating more hydrogen. The turnover frequency (TOF) per metal atom increases in the case of Au–Pd/TiO2 when compared to those of the monometallic catalysts (Au/TiO2 and Pd/TiO2 at similar loadings). In addition to hydrogen, during the initial period of the photo-reaction, acetaldehyde (in both the gas and dissolved phases) and methane (in the gas phase) are produced, suggesting that ethanol in aqueous solution reacted through dehydrogenation as well as C–C bond cleavage pathways. As the reaction proceeds, carbon dioxide is released following the same trend as that of methane. Results from this study are discussed in order to provide a comprehensive reaction network needed to understand the key parameters behind the photo-catalytic reaction on metal/semiconductor materials.
中文翻译:

在黑暗和光激发下Au,Pd和Au-Pd在乙醇对TiO 2的表面反应中作用的机理研究
原位红外光谱(FTIR)和催化反应用于研究乙醇在TiO 2和M / TiO 2(M = Au,Pd和Au-Pd)催化剂上的光氧化和光重整。研究了金属负载(在单金属和双金属系统中),是否存在气相氧以及水的作用的影响。Au–Pd / TiO 2样品的TEM图像表明,平均Au粒径为3.6 nm,而Pd为1.4 nm。在乙醇的光氧化过程中,表面上的反应中间体包括一氧化碳,乙醛,乙酸盐,甲酸盐和碳酸盐/碳酸氢盐类。乙醇脱氢产生的乙醛吸附的形成和稳定性的程度约为Au–Pd / TiO2 > Pd / TiO 2 > Au / TiO 2 > TiO 2。羧酸盐主要出现在所有催化剂上,而一氧化碳(在惰性气流下)和碳酸盐/碳酸氢盐(在O 2环境下)是专门在单金属和双金属体系中都含有较高Pd含量的催化剂上生产的。在乙醇/水存在下以4-5 mW cm -2的光通量(激发能= 3.44 eV)进行光催化制氢时,最大制氢速率为2.0×10 -4 mol min -1 g催化剂-1是用Au–Pd / TiO 2获得的催化剂(高于单金属催化剂)。UV-vis和X射线光电子能谱研究证明,双金属催化剂中Au的存在使Pd保持其金属状态,这归因于增强的载流子动力学,促进了电荷从Au转移至Pd,产生了更多的氢。与单金属催化剂(Au / TiO 2和Pd / TiO 2)相比,Au–Pd / TiO 2的情况下,每个金属原子的周转频率(TOF)增加在相似的负载下)。除了氢以外,在光反应的最初阶段,还会生成乙醛(在气相和溶解相中)和甲烷(在气相中),这表明水溶液中的乙醇通过脱氢以及C键裂解途径。随着反应的进行,二氧化碳的排放趋势与甲烷相同。为了提供一个全面的反应网络,以了解金属/半导体材料上的光催化反应背后的关键参数,需要对本研究的结果进行讨论。
更新日期:2017-11-14
中文翻译:

在黑暗和光激发下Au,Pd和Au-Pd在乙醇对TiO 2的表面反应中作用的机理研究
原位红外光谱(FTIR)和催化反应用于研究乙醇在TiO 2和M / TiO 2(M = Au,Pd和Au-Pd)催化剂上的光氧化和光重整。研究了金属负载(在单金属和双金属系统中),是否存在气相氧以及水的作用的影响。Au–Pd / TiO 2样品的TEM图像表明,平均Au粒径为3.6 nm,而Pd为1.4 nm。在乙醇的光氧化过程中,表面上的反应中间体包括一氧化碳,乙醛,乙酸盐,甲酸盐和碳酸盐/碳酸氢盐类。乙醇脱氢产生的乙醛吸附的形成和稳定性的程度约为Au–Pd / TiO2 > Pd / TiO 2 > Au / TiO 2 > TiO 2。羧酸盐主要出现在所有催化剂上,而一氧化碳(在惰性气流下)和碳酸盐/碳酸氢盐(在O 2环境下)是专门在单金属和双金属体系中都含有较高Pd含量的催化剂上生产的。在乙醇/水存在下以4-5 mW cm -2的光通量(激发能= 3.44 eV)进行光催化制氢时,最大制氢速率为2.0×10 -4 mol min -1 g催化剂-1是用Au–Pd / TiO 2获得的催化剂(高于单金属催化剂)。UV-vis和X射线光电子能谱研究证明,双金属催化剂中Au的存在使Pd保持其金属状态,这归因于增强的载流子动力学,促进了电荷从Au转移至Pd,产生了更多的氢。与单金属催化剂(Au / TiO 2和Pd / TiO 2)相比,Au–Pd / TiO 2的情况下,每个金属原子的周转频率(TOF)增加在相似的负载下)。除了氢以外,在光反应的最初阶段,还会生成乙醛(在气相和溶解相中)和甲烷(在气相中),这表明水溶液中的乙醇通过脱氢以及C键裂解途径。随着反应的进行,二氧化碳的排放趋势与甲烷相同。为了提供一个全面的反应网络,以了解金属/半导体材料上的光催化反应背后的关键参数,需要对本研究的结果进行讨论。


























 京公网安备 11010802027423号
京公网安备 11010802027423号