当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
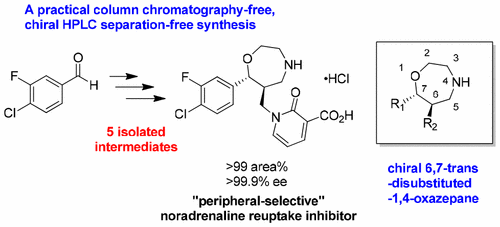
Development of a Practical Synthesis of a Peripherally Selective Noradrenaline Reuptake Inhibitor Possessing a Chiral 6,7-trans-Disubstituted-1,4-oxazepane as a Scaffold
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2017-11-13 00:00:00 , DOI: 10.1021/acs.oprd.7b00313 Kazuhisa Ishimoto 1 , Kotaro Yamaguchi 1 , Atsushi Nishimoto 1 , Mika Murabayashi 1 , Tomomi Ikemoto 1
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2017-11-13 00:00:00 , DOI: 10.1021/acs.oprd.7b00313 Kazuhisa Ishimoto 1 , Kotaro Yamaguchi 1 , Atsushi Nishimoto 1 , Mika Murabayashi 1 , Tomomi Ikemoto 1
Affiliation
|
A practical synthesis of a peripherally selective noradrenaline reuptake inhibitor that has a chiral 6,7-trans-disubstituted-1,4-oxazepane as a new class of scaffold is described. The amino alcohol possessing the desired stereochemistry was obtained with excellent dr and ee, starting from a commercially available aldehyde via a Morita–Baylis–Hillman reaction, Michael addition, isolation as maleic acid salt, reduction, and diastereomeric salt formation with (+)-10-camphorsulfonic acid. The desired single stereoisomer obtained at an early stage of the synthesis was used for seven-membered ring formation in fully telescoped processes, providing the chiral 6,7-trans-disubstituted-1,4-oxazepane efficiently. In addition to controls of dr and ee of the chiral 1,4-oxazepane, and control of N,O-selectivity in SN2 reaction of the intermediate mesylate with a pyridone derivative, finding appropriate intermediates that were amenable to isolation and upgrade of purity enabled a practical chiral HPLC separation-free, column chromatograph-free synthesis of the drug candidate with excellent chemical and optical purities in a higher overall yield.
中文翻译:

具有手性6,7-反式-双取代-1,4-恶唑烷的支架的周边选择性去甲肾上腺素再摄取抑制剂的实用合成的发展。
描述了具有手性6,7-反式-二取代-1,4-氧杂庚烷作为新型支架的外围选择性去甲肾上腺素再摄取抑制剂的实用合成。具有优异的立体化学的氨基醇是通过优异的dr和ee获得的,它是通过Morita-Baylis-Hillman反应,Michael加成,马来酸盐的分离,还原和与(+)-的非对映异构体盐的形成,从市场上可买到的醛中获得的。 10-樟脑磺酸。在合成的早期阶段获得的所需单一立体异构体在完全伸缩的过程中用于七元环的形成,从而有效地提供了手性6,7-反式-二取代-1,4-氧杂庚烷。除了控制手性1,4-恶唑烷的dr和ee以及控制中间体甲磺酸酯与吡啶酮衍生物在S N 2反应中的N,O选择性,找到适合分离和纯度提高的合适中间体,从而可以实现无手性HPLC分离,无柱色谱的药物候选物的实用合成。优良的化学和光学纯度,总收率更高。
更新日期:2017-11-13
中文翻译:

具有手性6,7-反式-双取代-1,4-恶唑烷的支架的周边选择性去甲肾上腺素再摄取抑制剂的实用合成的发展。
描述了具有手性6,7-反式-二取代-1,4-氧杂庚烷作为新型支架的外围选择性去甲肾上腺素再摄取抑制剂的实用合成。具有优异的立体化学的氨基醇是通过优异的dr和ee获得的,它是通过Morita-Baylis-Hillman反应,Michael加成,马来酸盐的分离,还原和与(+)-的非对映异构体盐的形成,从市场上可买到的醛中获得的。 10-樟脑磺酸。在合成的早期阶段获得的所需单一立体异构体在完全伸缩的过程中用于七元环的形成,从而有效地提供了手性6,7-反式-二取代-1,4-氧杂庚烷。除了控制手性1,4-恶唑烷的dr和ee以及控制中间体甲磺酸酯与吡啶酮衍生物在S N 2反应中的N,O选择性,找到适合分离和纯度提高的合适中间体,从而可以实现无手性HPLC分离,无柱色谱的药物候选物的实用合成。优良的化学和光学纯度,总收率更高。



























 京公网安备 11010802027423号
京公网安备 11010802027423号