当前位置:
X-MOL 学术
›
Integr. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A bioenergetic mechanism for amoeboid-like cell motility profiles tested in a microfluidic electrotaxis assay
Integrative Biology ( IF 2.5 ) Pub Date : 2017-09-29 00:00:00 , DOI: 10.1039/c7ib00086c Hagit Peretz-Soroka 1, 2, 3 , Reuven Tirosh 4, 5, 6, 7 , Jolly Hipolito 1, 2, 3 , Erwin Huebner 2, 3, 8, 9 , Murray Alexander 2, 3, 10, 11 , Jason Fiege 1, 2, 3 , Francis Lin 1, 2, 3, 8, 9
Integrative Biology ( IF 2.5 ) Pub Date : 2017-09-29 00:00:00 , DOI: 10.1039/c7ib00086c Hagit Peretz-Soroka 1, 2, 3 , Reuven Tirosh 4, 5, 6, 7 , Jolly Hipolito 1, 2, 3 , Erwin Huebner 2, 3, 8, 9 , Murray Alexander 2, 3, 10, 11 , Jason Fiege 1, 2, 3 , Francis Lin 1, 2, 3, 8, 9
Affiliation
|
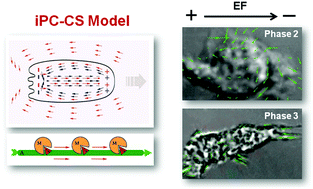
The amoeboid-like cell motility is known to be driven by the acidic enzymatic hydrolysis of ATP in the actin-myosin system. However, the electro-mechano-chemical coupling, whereby the free energy of ATP hydrolysis is transformed into the power of electrically polarized cell movement, is poorly understood. Previous experimental studies showed that actin filaments motion, cytoplasmic streaming, and muscle contraction can be reconstituted under actin-activated ATP hydrolysis by soluble non-filamentous myosin fragments. Thus, biological motility was demonstrated in the absence of a continuous protein network. These results lead to an integrative conceptual model for cell motility, which advocates an active role played by intracellular proton currents and cytoplasmic streaming (iPC-CS). In this model, we propose that protons and fluid currents develop intracellular electric polarization and pressure gradients, which generate an electro-hydrodynamic mode of amoeboid motion. Such energetic proton currents and active streaming are considered to be mainly driven by stereospecific ATP hydrolysis through myosin heads along oriented actin filaments. Key predictions of this model are supported by microscopy visualization and in-depth sub-population analysis of purified human neutrophils using a microfluidic electrotaxis assay. Three distinct phases in cell motility profiles, morphology, and cytoplasmic streaming in response to physiological ranges of chemoattractant stimulation and electric field application are revealed. Our results support an intrinsic electric dipole formation linked to different patterns of cytoplasmic streaming, which can be explained by the iPC-CS model. Collectively, this alternative biophysical mechanism of cell motility provides new insights into bioenergetics with relevance to potential new biomedical applications.
中文翻译:

在微流电分析法中测试的类淀粉样细胞运动特征的生物能机制
已知类肌纤维样细胞运动是由肌动蛋白-肌球蛋白系统中ATP的酸性酶水解作用驱动的。但是,人们对电-机械-化学耦合的理解是很少的,其中,ATP水解的自由能转化为电极化的细胞运动的力量。以前的实验研究表明,肌动蛋白丝的运动,细胞质流和肌肉收缩可以在肌动蛋白激活的ATP水解作用下通过可溶性非丝状肌球蛋白片段重建。因此,在不存在连续蛋白质网络的情况下证明了生物运动性。这些结果导致了细胞运动的综合概念模型,该模型提倡细胞内质子流和细胞质流(iPC-CS)发挥积极作用。在这个模型中 我们提出质子和流体流会发展细胞内的电极化和压力梯度,从而产生变形运动的电-流体动力学模式。这种高能质子流和活性流被认为主要是通过定向肌动蛋白丝通过肌球蛋白头部的立体有择ATP水解驱动的。显微镜可视化和使用微流电分析法对纯化的人类嗜中性粒细胞进行深入的亚群分析,可支持该模型的关键预测。揭示了细胞运动概况,形态学和胞质流响应化学趋化刺激和电场应用的生理范围的三个不同阶段。我们的结果支持固有的电偶极子形成与细胞质流的不同模式相关联,可以用iPC-CS模型来解释。总的来说,这种细胞运动的替代生物物理机制为与潜在的新生物医学应用相关的生物能学提供了新的见解。
更新日期:2017-11-13
中文翻译:

在微流电分析法中测试的类淀粉样细胞运动特征的生物能机制
已知类肌纤维样细胞运动是由肌动蛋白-肌球蛋白系统中ATP的酸性酶水解作用驱动的。但是,人们对电-机械-化学耦合的理解是很少的,其中,ATP水解的自由能转化为电极化的细胞运动的力量。以前的实验研究表明,肌动蛋白丝的运动,细胞质流和肌肉收缩可以在肌动蛋白激活的ATP水解作用下通过可溶性非丝状肌球蛋白片段重建。因此,在不存在连续蛋白质网络的情况下证明了生物运动性。这些结果导致了细胞运动的综合概念模型,该模型提倡细胞内质子流和细胞质流(iPC-CS)发挥积极作用。在这个模型中 我们提出质子和流体流会发展细胞内的电极化和压力梯度,从而产生变形运动的电-流体动力学模式。这种高能质子流和活性流被认为主要是通过定向肌动蛋白丝通过肌球蛋白头部的立体有择ATP水解驱动的。显微镜可视化和使用微流电分析法对纯化的人类嗜中性粒细胞进行深入的亚群分析,可支持该模型的关键预测。揭示了细胞运动概况,形态学和胞质流响应化学趋化刺激和电场应用的生理范围的三个不同阶段。我们的结果支持固有的电偶极子形成与细胞质流的不同模式相关联,可以用iPC-CS模型来解释。总的来说,这种细胞运动的替代生物物理机制为与潜在的新生物医学应用相关的生物能学提供了新的见解。


























 京公网安备 11010802027423号
京公网安备 11010802027423号