当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
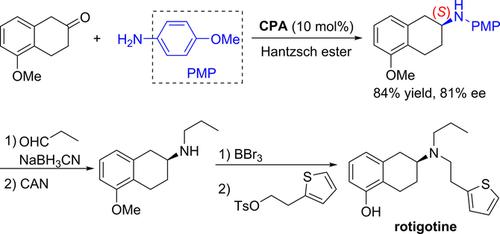
Enantioselective Synthesis of β‐Aminotetralins via Chiral Phosphoric Acid‐catalyzed Reductive Amination of β‐Tetralones
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2017-11-29 , DOI: 10.1002/adsc.201701198 Do Young Park 1 , Kyung-Hee Kim 1 , Cheol-Hong Cheon 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2017-11-29 , DOI: 10.1002/adsc.201701198 Do Young Park 1 , Kyung-Hee Kim 1 , Cheol-Hong Cheon 1
Affiliation
|
A new protocol for the synthesis of chiral β‐aminotetralins has been developed via chiral phosphoric acid‐catalyzed asymmetric reductive amination of β‐tetralones using a Hantzsch ester as an organic hydride donor. Various chiral β‐aminotetralins were obtained in good yields with good to high enantioselectivities. Furthermore, the utility of our new protocol was successfully demonstrated in the enantioselective synthesis of rotigotine.
中文翻译:

手性磷酸催化β-四氢萘酮的还原胺化反应,对映体合成β-氨基四氢萘
通过使用Hantzsch酯作为有机氢化物供体,通过手性磷酸催化β-四氢萘酮的不对称还原胺化反应,开发了一种合成手性β-氨基四氢萘的新方案。以高收率和良好至高对映选择性获得了各种手性β-氨基四氢萘。此外,我们的新协议的效用已在罗替戈汀的对映选择性合成中得到了成功证明。
更新日期:2017-11-29
中文翻译:

手性磷酸催化β-四氢萘酮的还原胺化反应,对映体合成β-氨基四氢萘
通过使用Hantzsch酯作为有机氢化物供体,通过手性磷酸催化β-四氢萘酮的不对称还原胺化反应,开发了一种合成手性β-氨基四氢萘的新方案。以高收率和良好至高对映选择性获得了各种手性β-氨基四氢萘。此外,我们的新协议的效用已在罗替戈汀的对映选择性合成中得到了成功证明。



























 京公网安备 11010802027423号
京公网安备 11010802027423号