当前位置:
X-MOL 学术
›
WIREs Comput. Mol. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Energy decomposition analysis
Wiley Interdisciplinary Reviews: Computational Molecular Science ( IF 11.4 ) Pub Date : 2017-11-10 , DOI: 10.1002/wcms.1345 Lili Zhao 1 , Moritz von Hopffgarten 2 , Diego M. Andrada 2 , Gernot Frenking 1, 2, 3
Wiley Interdisciplinary Reviews: Computational Molecular Science ( IF 11.4 ) Pub Date : 2017-11-10 , DOI: 10.1002/wcms.1345 Lili Zhao 1 , Moritz von Hopffgarten 2 , Diego M. Andrada 2 , Gernot Frenking 1, 2, 3
Affiliation
|
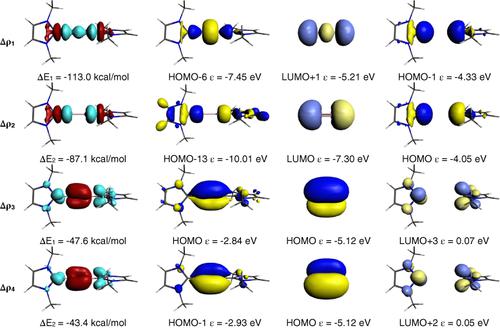
The energy decomposition analysis (EDA) is a powerful method for a quantitative interpretation of chemical bonds in terms of three major components. The instantaneous interaction energy ΔEint between two fragments A and B in a molecule A–B is partitioned in three terms, namely (1) the quasiclassical electrostatic interaction ΔEelstat between the fragments; (2) the repulsive exchange (Pauli) interaction ΔEPauli between electrons of the two fragments having the same spin, and (3) the orbital (covalent) interaction ΔEorb which comes from the orbital relaxation and the orbital mixing between the fragments. The latter term can be decomposed into contributions of orbitals with different symmetry which makes it possible to distinguish between σ, π, and δ bonding. After a short introduction into the theoretical background of the EDA we present illustrative examples of main group and transition metal chemistry. The results show that the EDA terms can be interpreted in chemically meaningful way thus providing a bridge between quantum chemical calculations and heuristic bonding models of traditional chemistry. The extension to the EDA–Natural Orbitals for Chemical Valence (NOCV) method makes it possible to breakdown the orbital term ΔEorb into pairwise orbital contributions of the interacting fragments. The method provides a bridge between MO correlations diagrams and pairwise orbital interactions, which have been shown in the past to correlate with the structures and reactivities of molecules. There is a link between frontier orbital theory and orbital symmetry rules and the quantitative charge‐ and energy partitioning scheme that is provided by the EDA–NOCV terms. The strength of the pairwise orbital interactions can quantitatively be estimated and the associated change in the electronic structure can be visualized by plotting the deformation densities.
中文翻译:

能量分解分析
能量分解分析(EDA)是从三个主要方面定量解释化学键的有力方法。分子A–B中两个片段A和B之间的瞬时相互作用能ΔE int被划分为三项,即(1)片段之间的准经典静电相互作用ΔE elstat;(2)具有相同自旋的两个片段的电子之间的排斥交换(Pauli)相互作用ΔE Pauli,以及(3)轨道(共价)相互作用ΔE orb这来自碎片之间的轨道弛豫和轨道混合。后一项可以分解为具有不同对称性的轨道的贡献,从而可以区分σ键,π键和δ键。在简要介绍了EDA的理论背景之后,我们给出了主要基团和过渡金属化学的示例性例子。结果表明,EDA术语可以用化学上有意义的方式进行解释,从而在量子化学计算和传统化学的启发式键合模型之间架起了桥梁。EDA-化学价自然轨道(NOCV)方法的扩展使分解轨道项ΔE orb成为可能成相互作用的碎片的成对轨道贡献。该方法提供了MO相关图和成对轨道相互作用之间的桥梁,过去已经证明该关系与分子的结构和反应性相关。前沿轨道理论和轨道对称规则与EDA–NOCV术语提供的定量电荷和能量分配方案之间存在联系。可以定量地估计成对轨道相互作用的强度,并且可以通过绘制变形密度来可视化电子结构中的相关变化。
更新日期:2017-11-10
中文翻译:

能量分解分析
能量分解分析(EDA)是从三个主要方面定量解释化学键的有力方法。分子A–B中两个片段A和B之间的瞬时相互作用能ΔE int被划分为三项,即(1)片段之间的准经典静电相互作用ΔE elstat;(2)具有相同自旋的两个片段的电子之间的排斥交换(Pauli)相互作用ΔE Pauli,以及(3)轨道(共价)相互作用ΔE orb这来自碎片之间的轨道弛豫和轨道混合。后一项可以分解为具有不同对称性的轨道的贡献,从而可以区分σ键,π键和δ键。在简要介绍了EDA的理论背景之后,我们给出了主要基团和过渡金属化学的示例性例子。结果表明,EDA术语可以用化学上有意义的方式进行解释,从而在量子化学计算和传统化学的启发式键合模型之间架起了桥梁。EDA-化学价自然轨道(NOCV)方法的扩展使分解轨道项ΔE orb成为可能成相互作用的碎片的成对轨道贡献。该方法提供了MO相关图和成对轨道相互作用之间的桥梁,过去已经证明该关系与分子的结构和反应性相关。前沿轨道理论和轨道对称规则与EDA–NOCV术语提供的定量电荷和能量分配方案之间存在联系。可以定量地估计成对轨道相互作用的强度,并且可以通过绘制变形密度来可视化电子结构中的相关变化。


























 京公网安备 11010802027423号
京公网安备 11010802027423号