Molecular Catalysis ( IF 4.6 ) Pub Date : 2017-11-06 , DOI: 10.1016/j.mcat.2017.09.023 Xiang Li , Kaixuan Zhang , Li Qin , Haihong Ma
|
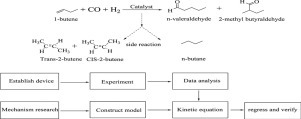
The kinetic studies of hydroformylation of 1-butene to produce pentanal were investigated by using Rh/PPh3 complex as homogeneous catalyst, valeraldehyde and its polycondensates as solvent. The experiment was carried out on small hydroformylation continuous reaction installation. The effects of reaction temperature, the concentration of 1-butene, the partial pressure of CO or H2 and the concentration of rhodium of catalyst on the reaction rate were studied. Based on the reaction mechanism of hydroformylation and the latest research results of hydroformylation kinetics, the kinetic equation suitable for the hydroformylation of 1-butene was established. The parameters of the kinetic equation were regressed and verified with the experimental data. The relationship between the normal/isomeric ratio of pentanal and the partial pressure of CO was further explored.
中文翻译:

均相Rh / PPh 3络合物催化剂催化1-丁烯加氢甲酰化的动力学研究
以Rh / PPh 3络合物为均相催化剂,戊醛及其缩合物为溶剂,研究了1-丁烯加氢甲酰化制戊醛的动力学。该实验是在小型加氢甲酰化连续反应装置上进行的。反应温度,1-丁烯浓度,CO或H 2分压的影响研究了催化剂的铑浓度对反应速率的影响。基于加氢甲酰化的反应机理和加氢甲酰化动力学的最新研究成果,建立了适合1-丁烯加氢甲酰化反应的动力学方程。对动力学方程的参数进行回归,并与实验数据进行验证。进一步探讨了戊醛的正/异构比与CO分压之间的关系。


























 京公网安备 11010802027423号
京公网安备 11010802027423号