Journal of Catalysis ( IF 7.3 ) Pub Date : 2017-11-06 , DOI: 10.1016/j.jcat.2017.10.020 Xiaohua Zhang , Ping Lu , Chen Zhang , Xiangzhi Cui , Yingfeng Xu , Haiyun Qu , Jianlin Shi
|
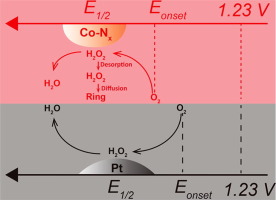
M-Nx/C (M = Fe, Co) type electro-catalyst as a promising alternative to Pt-based electro-catalyst for oxygen reduction reaction (ORR) in fuel cells has been studied for years. However, the mechanism of this four-electron process involving several successive steps and the dynamic intermediate (hydrogen peroxide) participation still remains obscure. In this study, a series of Co-Nx/C with varied densities of Co-Nx sites have been obtained for probing the ORR activity and pathway in acid media via adopting zinc ions as a size-comparable template to disperse and regulate Co-Nx sites. Importantly, it has been found that the half-wave potential can be positively correlated with the weight percentage of ionic cobalt species, which suggests the decisive role of the density of Co-Nx sites on ORR activity. More importantly, our results suggest that both Co-Nx/C and 20 wt% Pt/C catalyze ORR via two successive steps: ORR begins at the onset potential accompanying the generation of H2O2 intermediate, which is more easily to be adsorbed on platinum than on Co-Nx/C surface as detected by ring electrode; Subsequently at the half-wave potential and more negative, H2O2 is further reduced immediately and efficiently on platinum, but unfortunately at much lower rate on Co-Nx/C. Thus in addition to improving the initial ORR activity by maximizing the M-Nx coordination, it will be of great significance to endow the non-platinum catalysts with surface active sites capable of adsorbing and efficiently reducing the H2O2 intermediate for the complete oxygen reduction to water.
中文翻译:

理解酸性介质中MN x / C电催化剂的ORR活性和电子转移途径
MN x / C(M = Fe,Co)型电催化剂作为燃料电池中氧还原反应(ORR)的Pt基电催化剂的有前途的替代品,已经进行了多年研究。但是,这种涉及多个连续步骤的四电子过程的机理以及动态中间产物(过氧化氢)的参与仍然不清楚。在这项研究中,通过采用锌离子作为可比尺寸的模板分散和调节Co ,获得了一系列具有不同密度Co-N x位点的Co-N x / C,用于探测酸介质中的ORR活性和途径。-N x网站。重要的是,已发现半波电势与离子钴物种的重量百分比呈正相关,这表明Co-N x位点密度对ORR活性具有决定性作用。更重要的是,我们的结果表明Co-N x / C和20 wt%Pt / C都通过两个连续步骤来催化ORR :ORR从伴随H 2 O 2中间体生成的起始电位开始。环形电极检测到,与Co-N x / C表面相比,吸附在铂上的吸附量更高;随后在半波电势和更多负值处,H 2 O 2在铂上可以立即有效地进一步降低汞的含量,但不幸的是在Co-N x / C上速率要低得多。因此,除了通过最大程度地提高MN x配位来提高初始ORR活性外,赋予非铂催化剂表面活性位能够吸附并有效还原H 2 O 2中间体以完全还原氧也具有重要意义。浇水。


























 京公网安备 11010802027423号
京公网安备 11010802027423号