Applied Catalysis B: Environment and Energy ( IF 22.1 ) Pub Date : 2017-11-07 , DOI: 10.1016/j.apcatb.2017.11.014 Edyta Tabor , Galina Sádovská , Milan Bernauer , Petr Sazama , Jana Nováková , Vlastimil Fíla , Tomáš Kmječ , Jaroslav Kohout , Karel Závěta , Zdeněk Sobalík
|
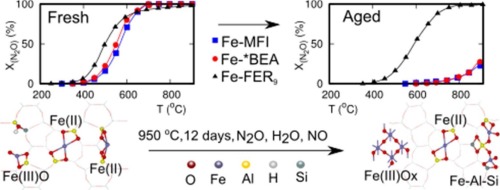
The long-term stabilities of a group of iron zeolites with MFI, *BEA, and FER structures and similar Fe/Al ratios were evaluated to assess their performance as catalysts for N2O decomposition. Conditions relevant for the application of the catalyst at the secondary level for N2O elimination, i.e., directly after the NH3 oxidation step of the process of nitric acid production, were investigated. These conditions included reaction temperatures of up to 900 °C and the presence of high concentrations of water vapour, oxygen, and NO. The focus of the study was the comparison of the MFI and *BEA zeolites with the FER-based zeolite, a catalyst established as relatively stable under such conditions. The structural analysis of the individual zeolite frameworks and iron species involved a combination of several methods, and provided insight into the framework modification as well as identification and semiquantitative determination of the individual iron species. In the zeolite catalysts, these iron species were in the form of either isolated Fe(II) and Fe(III)-oxo ions, polynuclear Fe(III)-oxo complexes, or small Fe oxide particles. None of the zeolite structures displayed a high extent of structural collapse of the framework; thus, structural collapse does not explain the observed decrease in activity. An investigation of the kinetics of the N2O decomposition, both before and after ageing under relevant reaction conditions, proved the dominant role of isolated Fe(II) ions that were accessible to the reacting gas-phase molecules. The kinetic study also identified the differences in the ability of the three zeolites to stabilize the active sites in individual framework types during long-term exposure to challenging reaction conditions. It has been shown that, while the isolated Fe(II) ions were present in adequate concentration for N2O decomposition activity at the relevant temperature region in all three zeolite-based catalysts, the MFI and *BEA zeolites were not able to effectively stabilize the active iron structure during ageing for over 12 days. In contrast, the FER framework survived a test for over 24 days. The iron species formed in MFI and *BEA zeolites during ageing were mostly large FeOx species and displayed very low activity that approached the activity of FeOx clusters supported on SiO2.
中文翻译:

在实际生产硝酸技术中,将铁沸石用于N 2 O高温分解的可行性
评估了具有MFI,* BEA和FER结构以及相似的Fe / Al比的一组铁沸石的长期稳定性,以评估其作为N 2 O分解催化剂的性能。与在二级去除N 2 O时(即紧接在NH 3之后)施加催化剂有关的条件对硝酸生产过程中的氧化步骤进行了研究。这些条件包括最高900°C的反应温度以及高浓度的水蒸气,氧气和NO的存在。研究的重点是将MFI和* BEA沸石与FER型沸石进行比较,FER型沸石在这种条件下被确定为相对稳定。单个沸石骨架和铁物种的结构分析涉及多种方法的组合,并提供了对骨架改性以及单个铁物种的鉴定和半定量测定的见识。在沸石催化剂中,这些铁物质的形式为分离的Fe(II)和Fe(III)-氧代离子,多核Fe(III)-氧代配合物或小的Fe氧化物颗粒。没有一种沸石结构显示出框架的高度结构塌陷。因此,结构崩溃不能解释观察到的活性降低。氮的动力学研究在相关反应条件下老化之前和之后的2 O分解证明了反应的气相分子可接近的离析的Fe(II)离子的主要作用。动力学研究还确定了在长期暴露于具有挑战性的反应条件期间,三种沸石稳定各个构架类型的活性位的能力的差异。结果表明,虽然分离出的Fe(II)离子以足够的浓度存在于N 2中在所有三种基于沸石的催化剂,MFI和* BEA沸石中,在相关温度区域的O分解活性均无法在超过12天的老化过程中有效地稳定活性铁的结构。相比之下,FER框架在测试中存活了超过24天。在时效过程中,MFI和* BEA沸石中形成的铁物种主要是大的FeO x物种,并且显示出非常低的活性,接近SiO 2负载的FeO x簇的活性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号