当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Reaction pathway for partial hydrogenation of 1,3-butadiene over Pt/SiO2
Catalysis Science & Technology ( IF 5 ) Pub Date : 2017-10-30 00:00:00 , DOI: 10.1039/c7cy01882g Chaoquan Hu 1, 2, 3, 4, 5 , Jiahan Sun 1, 2, 3, 4, 5 , Yafeng Yang 1, 2, 3, 4, 5 , Qingshan Zhu 1, 2, 3, 4, 5 , Bin Yu 5, 6, 7
Catalysis Science & Technology ( IF 5 ) Pub Date : 2017-10-30 00:00:00 , DOI: 10.1039/c7cy01882g Chaoquan Hu 1, 2, 3, 4, 5 , Jiahan Sun 1, 2, 3, 4, 5 , Yafeng Yang 1, 2, 3, 4, 5 , Qingshan Zhu 1, 2, 3, 4, 5 , Bin Yu 5, 6, 7
Affiliation
|
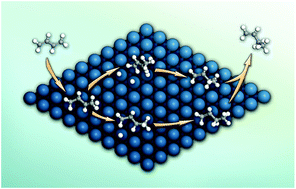
The reaction pathway for partial hydrogenation of 1,3-butadiene over a Pt/SiO2 catalyst was explored with a combination of in situ diffuse reflectance infrared Fourier transform (DRIFT) spectroscopy, intrinsic kinetics, and density functional theory (DFT) calculations. Under the present experimental conditions, the catalyst displayed a nearly constant product composition with ∼97% selectivity to butenes. In situ DRIFT characterization revealed that the 1-buten-3-yl radical (1B3R), generated from the addition of one hydrogen atom to a terminal carbon of 1,3-butadiene, was the dominant intermediate in the partial hydrogenation of 1,3-butadiene. Kinetic analysis showed that the hydrogenation of 1B3R was the rate-determining step in the formation of butenes. Based on the above experimental results, DFT calculations were employed to investigate the reaction pathway with 1B3R as the intermediate on a Pt(111) surface. Interestingly, it was found that 1B3R can be easily formed from the hydrogenation of 1,3-butadiene with a di-σ configuration rather than the most stable tetra-σ structure on the Pt(111) surface. This hydrogenation step occurs between the non-coordinated terminal carbon and a hydrogen atom on a top site with an energy barrier of 23.2 kJ mol−1. The second hydrogenation step from 1B3R to butenes requires relatively higher activation barriers to proceed, being consistent with the experimental kinetics. Finally, the selectivity order for butenes and the structure sensitivity of Pt-catalyzed partial hydrogenation of 1,3-butadiene were discussed.
中文翻译:

Pt / SiO 2上1,3-丁二烯部分加氢的反应途径
结合原位漫反射红外傅里叶变换(DRIFT)光谱,内在动力学和密度泛函理论(DFT)计算,探索了在Pt / SiO 2催化剂上1,3-丁二烯部分加氢的反应途径。在目前的实验条件下,该催化剂显示出几乎恒定的产物组成,对丁烯的选择性为〜97%。原位DRIFT表征表明,由一个氢原子加成到1,3-丁二烯的末端碳上而生成的1-buten-3-yl自由基(1B3R)是1,3-丁二烯部分加氢的主要中间体。 。动力学分析表明,1B3R的氢化是丁烯形成过程中的决定速率的步骤。基于以上实验结果,采用DFT计算来研究以1B3R为中间物在Pt(111)表面上的反应途径。有趣的是,发现1B3R可以很容易地由具有di-σ构型而不是Pt(111)表面上最稳定的四σ结构的1,3-丁二烯的氢化反应形成。该氢化步骤发生在非配位末端碳与顶部位点上的氢原子之间,能量垒为23.2 kJ mol-1。从1B3R到丁烯的第二个氢化步骤需要相对较高的活化势垒才能进行,这与实验动力学一致。最后,讨论了丁烯的选择性顺序和Pt催化1,3-丁二烯部分加氢的结构敏感性。
更新日期:2017-11-10
中文翻译:

Pt / SiO 2上1,3-丁二烯部分加氢的反应途径
结合原位漫反射红外傅里叶变换(DRIFT)光谱,内在动力学和密度泛函理论(DFT)计算,探索了在Pt / SiO 2催化剂上1,3-丁二烯部分加氢的反应途径。在目前的实验条件下,该催化剂显示出几乎恒定的产物组成,对丁烯的选择性为〜97%。原位DRIFT表征表明,由一个氢原子加成到1,3-丁二烯的末端碳上而生成的1-buten-3-yl自由基(1B3R)是1,3-丁二烯部分加氢的主要中间体。 。动力学分析表明,1B3R的氢化是丁烯形成过程中的决定速率的步骤。基于以上实验结果,采用DFT计算来研究以1B3R为中间物在Pt(111)表面上的反应途径。有趣的是,发现1B3R可以很容易地由具有di-σ构型而不是Pt(111)表面上最稳定的四σ结构的1,3-丁二烯的氢化反应形成。该氢化步骤发生在非配位末端碳与顶部位点上的氢原子之间,能量垒为23.2 kJ mol-1。从1B3R到丁烯的第二个氢化步骤需要相对较高的活化势垒才能进行,这与实验动力学一致。最后,讨论了丁烯的选择性顺序和Pt催化1,3-丁二烯部分加氢的结构敏感性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号