当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Heterogeneous Ni- and Cd-Bearing Ferrihydrite Precipitation and Recrystallization on Quartz under Acidic pH Condition
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2017-11-09 00:00:00 , DOI: 10.1021/acsearthspacechem.7b00097 Chong Dai 1 , Mingxi Lin 1 , Yandi Hu 1
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2017-11-09 00:00:00 , DOI: 10.1021/acsearthspacechem.7b00097 Chong Dai 1 , Mingxi Lin 1 , Yandi Hu 1
Affiliation
|
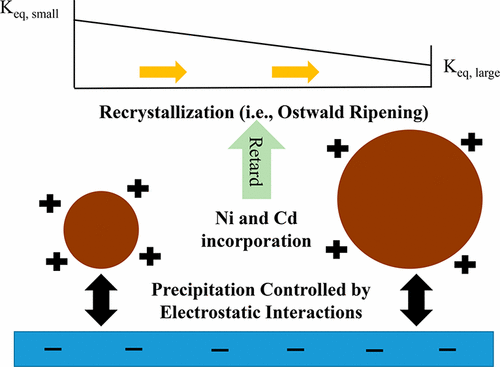
Ferrihydrite, as one of the most common naturally occurring iron oxides, can sequester toxic metals through co-precipitation. In this study, using grazing-incidence small-angle X-ray scattering, the heterogeneous precipitation of pure, Ni-, and Cd-bearing ferrihydrite on quartz was quantified in 0.1 mM Fe3+ solutions in the absence and presence of 1 mM Ni2+ or Cd2+ (pH 3.8 ± 0.1). Under acidic condition, the limited hydrolysis of metal ions resulted in their small amounts of incorporation in ferrihydrite lattices (<0.1%), several orders of magnitude lower than those reported at neutral and alkaline pH conditions. The presence of Ni2+ or Cd2+ did not significantly affect the surface charges of either ferrihydrite pre-nucleation clusters (PNCs) or quartz surfaces. Therefore, with similar electrostatic interactions between PNCs and quartz, similar initial heterogeneous precipitation rates of pure and Ni- and Cd-bearing ferrihydrite on quartz were observed. Later on, continuous heterogeneous nucleation and growth of ferrihydrite nanoparticles resulted in their increased polydispersity, and the size-dependent solubility of ferrihydrite nanoparticles caused the Ostwald ripening process. The presence of Ni and Cd was found to retard the recrystallization of ferrihydrite, probably as a result of their structural incorporation, which could inhibit the dissolution of ferrihydrite. This study provided new kinetic and mechanistic insights for understanding the effects of metal ions on the heterogeneous precipitation and recrystallization processes of ferrihydrite nanoparticles on mineral surfaces, which can better predict the fate and transport of heavy metals.
中文翻译:

酸性pH条件下在石英上非均相含Ni和Cd的水铁矿的沉淀和重结晶
水铁矿是最常见的天然铁氧化物之一,可以通过共沉淀来隔离有毒金属。在这项研究中,使用掠入射小角度X射线散射,在不存在和存在1 mM Ni的情况下,在0.1 mM Fe 3+溶液中对石英上纯净,Ni和Cd含铁水铁矿的非均相沉淀进行了定量。2+或Cd 2+(pH 3.8±0.1)。在酸性条件下,金属离子的有限水解导致它们少量掺入三水铁矿晶格(<0.1%),比在中性和碱性pH条件下所报道的离子数低几个数量级。Ni 2+或Cd 2+的存在不会显着影响水铁矿预成核簇(PNC)或石英表面的表面电荷。因此,在PNC与石英之间存在相似的静电相互作用的情况下,观察到石英上纯净的,含Ni和Cd的亚铁水合物的初始初始非均相沉淀速率。后来,水铁矿纳米颗粒的连续异质成核和生长导致它们的多分散性增加,并且铁氧体纳米颗粒的尺寸依赖性溶解度引起了奥斯特瓦尔德熟化过程。发现Ni和Cd的存在可能阻碍了水铁矿的重结晶,这可能是由于它们的结构结合所致,其可以抑制水铁矿的溶解。
更新日期:2017-11-09
中文翻译:

酸性pH条件下在石英上非均相含Ni和Cd的水铁矿的沉淀和重结晶
水铁矿是最常见的天然铁氧化物之一,可以通过共沉淀来隔离有毒金属。在这项研究中,使用掠入射小角度X射线散射,在不存在和存在1 mM Ni的情况下,在0.1 mM Fe 3+溶液中对石英上纯净,Ni和Cd含铁水铁矿的非均相沉淀进行了定量。2+或Cd 2+(pH 3.8±0.1)。在酸性条件下,金属离子的有限水解导致它们少量掺入三水铁矿晶格(<0.1%),比在中性和碱性pH条件下所报道的离子数低几个数量级。Ni 2+或Cd 2+的存在不会显着影响水铁矿预成核簇(PNC)或石英表面的表面电荷。因此,在PNC与石英之间存在相似的静电相互作用的情况下,观察到石英上纯净的,含Ni和Cd的亚铁水合物的初始初始非均相沉淀速率。后来,水铁矿纳米颗粒的连续异质成核和生长导致它们的多分散性增加,并且铁氧体纳米颗粒的尺寸依赖性溶解度引起了奥斯特瓦尔德熟化过程。发现Ni和Cd的存在可能阻碍了水铁矿的重结晶,这可能是由于它们的结构结合所致,其可以抑制水铁矿的溶解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号