当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Halide Control of N,N-Coordination versus N,C-Cyclometalation and Stereospecific Phenyl Ring Deuteration of Osmium(II) p-Cymene Phenylazobenzothiazole Complexes
Organometallics ( IF 2.8 ) Pub Date : 2017-11-09 00:00:00 , DOI: 10.1021/acs.organomet.7b00501 Russell J Needham 1 , Abraha Habtemariam 1 , Nicolas P E Barry 1, 2 , Guy Clarkson 1 , Peter J Sadler 1
Organometallics ( IF 2.8 ) Pub Date : 2017-11-09 00:00:00 , DOI: 10.1021/acs.organomet.7b00501 Russell J Needham 1 , Abraha Habtemariam 1 , Nicolas P E Barry 1, 2 , Guy Clarkson 1 , Peter J Sadler 1
Affiliation
|
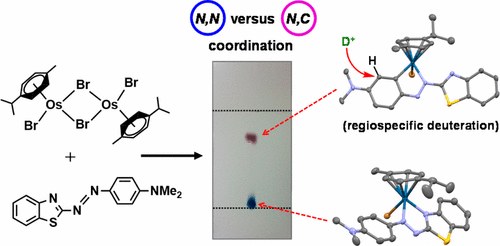
We report the synthesis of halido Os(II) p-cymene complexes bearing bidentate chelating phenylazobenzothiazole (AZBTZ) ligands. Unlike the analogous phenylazopyridine (AZPY) complexes, AZBTZ-NMe2 is capable of both N,N-coordination to Os(II) and cyclometalation to form N,C-coordinated species. N,C-Coordination occurs via an azo nitrogen and an ortho carbon on the aniline ring, as identified by 1H NMR and X-ray crystallography of [Os(p-cym)(N,N-AZBTZ-NMe2)Cl]PF6 (1a), [Os(p-cym)(N,N-AZBTZ-NMe2)Br]PF6 (2a), [Os(p-cym)(N,C-AZBTZ-NMe2)Br] (2b), and [Os(p-cym)(N,C-AZBTZ-NMe2)I] (3b). The N,C-coordinated species is more stable and is not readily converted to the N,N-coordinated complex. Analysis of the crystal structures suggests that their formation is influenced by steric interactions between the p-cym and AZBTZ-NMe2 ligands: in particular, larger monodentate halide ligands favor N,C-coordination. The complexes [Os(p-cym)(N,N-Me2-AZBTZ-NH2)Cl]PF6 (4) and [Os(p-cym)(N,N-Me2-AZBTZ-NH2)I]PF6 (5) were synthesized with methyl groups blocking the ortho positions on the aniline ring, forcing an N,N-coordination geometry. 1H NMR NOE experiments confirmed hindered rotation of the arene ligand and steric crowding around the metal center. Complex 2b exhibited unexpected behavior under acidic conditions, involving regiospecific deuteration of the aniline ring at the meta position, as observed by 1H NMR and high-resolution ESI-MS. Deuterium exchange occurs only under acidic conditions, suggesting an associative mechanism. The calculated partial charges on 2b show that the meta carbon is significantly more negatively charged, which may account for the regiospecificity of deuterium exchange.
中文翻译:

锇(II)对伞花烃苯基苯并噻唑配合物的N,N-配位与N,C-环金属化和立体特异性苯环氘化的卤化物控制
我们报道了带有二齿螯合苯基偶氮苯并噻唑 (AZBTZ) 配体的卤代 Os(II) 对伞花烃配合物的合成。与类似的苯基偶氮吡啶 (AZPY) 配合物不同,AZBTZ-NMe 2能够与 Os(II) 进行N,N配位,并能够进行环金属化以形成N,C配位物质。N,C-配位通过苯胺环上的偶氮氮和邻位碳发生,如[Os( p -cym)( N,N -AZBTZ-NMe 2 )Cl]的1 H NMR 和 X 射线晶体学所鉴定。 PF 6 ( 1a ), [Os( p -cym)( N,N -AZBTZ-NMe 2 )Br]PF 6 ( 2a ), [Os( p -cym)( N,C -AZBTZ-NMe 2 )Br] ( 2b )和[Os( p -cym)( N,C -AZBTZ-NMe 2 )I]( 3b )。N ,C-配位物质更稳定并且不容易转化为N,N-配位络合物。晶体结构的分析表明它们的形成受到p -cym和AZBTZ-NMe 2配体之间的空间相互作用的影响:特别是,较大的单齿卤化物配体有利于N,C-配位。配合物 [Os( p -cym)( N,N -Me 2 -AZBTZ-NH 2 )Cl]PF 6 ( 4 ) 和 [Os( p -cym)( N,N -Me 2 -AZBTZ-NH 2 ) I]PF 6 ( 5 ) 是通过甲基基团封闭苯胺环上的邻位来合成的,从而形成N,N -配位几何形状。1 H NMR NOE 实验证实了芳烃配体的旋转受阻以及金属中心周围的空间拥挤。通过1 H NMR 和高分辨率 ESI-MS观察到,配合物2b在酸性条件下表现出意想不到的行为,包括间位苯胺环的区域特异性氘化。氘交换仅在酸性条件下发生,表明存在缔合机制。计算出的2b部分电荷表明,间位碳的负电荷明显更多,这可能解释了氘交换的区域特异性。
更新日期:2017-11-09
中文翻译:

锇(II)对伞花烃苯基苯并噻唑配合物的N,N-配位与N,C-环金属化和立体特异性苯环氘化的卤化物控制
我们报道了带有二齿螯合苯基偶氮苯并噻唑 (AZBTZ) 配体的卤代 Os(II) 对伞花烃配合物的合成。与类似的苯基偶氮吡啶 (AZPY) 配合物不同,AZBTZ-NMe 2能够与 Os(II) 进行N,N配位,并能够进行环金属化以形成N,C配位物质。N,C-配位通过苯胺环上的偶氮氮和邻位碳发生,如[Os( p -cym)( N,N -AZBTZ-NMe 2 )Cl]的1 H NMR 和 X 射线晶体学所鉴定。 PF 6 ( 1a ), [Os( p -cym)( N,N -AZBTZ-NMe 2 )Br]PF 6 ( 2a ), [Os( p -cym)( N,C -AZBTZ-NMe 2 )Br] ( 2b )和[Os( p -cym)( N,C -AZBTZ-NMe 2 )I]( 3b )。N ,C-配位物质更稳定并且不容易转化为N,N-配位络合物。晶体结构的分析表明它们的形成受到p -cym和AZBTZ-NMe 2配体之间的空间相互作用的影响:特别是,较大的单齿卤化物配体有利于N,C-配位。配合物 [Os( p -cym)( N,N -Me 2 -AZBTZ-NH 2 )Cl]PF 6 ( 4 ) 和 [Os( p -cym)( N,N -Me 2 -AZBTZ-NH 2 ) I]PF 6 ( 5 ) 是通过甲基基团封闭苯胺环上的邻位来合成的,从而形成N,N -配位几何形状。1 H NMR NOE 实验证实了芳烃配体的旋转受阻以及金属中心周围的空间拥挤。通过1 H NMR 和高分辨率 ESI-MS观察到,配合物2b在酸性条件下表现出意想不到的行为,包括间位苯胺环的区域特异性氘化。氘交换仅在酸性条件下发生,表明存在缔合机制。计算出的2b部分电荷表明,间位碳的负电荷明显更多,这可能解释了氘交换的区域特异性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号