当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Discovery and Optimization of Potent, Cell-Active Pyrazole-Based Inhibitors of Lactate Dehydrogenase (LDH)
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2017-11-09 00:00:00 , DOI: 10.1021/acs.jmedchem.7b00941 Ganesha Rai 1 , Kyle R Brimacombe 1 , Bryan T Mott 1 , Daniel J Urban 1 , Xin Hu 1 , Shyh-Ming Yang 1 , Tobie D Lee 1 , Dorian M Cheff 1 , Jennifer Kouznetsova 1 , Gloria A Benavides 2 , Katie Pohida 1 , Eric J Kuenstner 1 , Diane K Luci 1 , Christine M Lukacs 3 , Douglas R Davies 3 , David M Dranow 3 , Hu Zhu 1 , Gary Sulikowski 4 , William J Moore 5 , Gordon M Stott 5 , Andrew J Flint 5 , Matthew D Hall 1 , Victor M Darley-Usmar 2 , Leonard M Neckers 6 , Chi V Dang 7 , Alex G Waterson 4 , Anton Simeonov 1 , Ajit Jadhav 1 , David J Maloney 1
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2017-11-09 00:00:00 , DOI: 10.1021/acs.jmedchem.7b00941 Ganesha Rai 1 , Kyle R Brimacombe 1 , Bryan T Mott 1 , Daniel J Urban 1 , Xin Hu 1 , Shyh-Ming Yang 1 , Tobie D Lee 1 , Dorian M Cheff 1 , Jennifer Kouznetsova 1 , Gloria A Benavides 2 , Katie Pohida 1 , Eric J Kuenstner 1 , Diane K Luci 1 , Christine M Lukacs 3 , Douglas R Davies 3 , David M Dranow 3 , Hu Zhu 1 , Gary Sulikowski 4 , William J Moore 5 , Gordon M Stott 5 , Andrew J Flint 5 , Matthew D Hall 1 , Victor M Darley-Usmar 2 , Leonard M Neckers 6 , Chi V Dang 7 , Alex G Waterson 4 , Anton Simeonov 1 , Ajit Jadhav 1 , David J Maloney 1
Affiliation
|
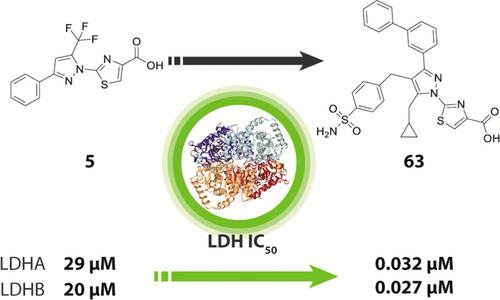
We report the discovery and medicinal chemistry optimization of a novel series of pyrazole-based inhibitors of human lactate dehydrogenase (LDH). Utilization of a quantitative high-throughput screening paradigm facilitated hit identification, while structure-based design and multiparameter optimization enabled the development of compounds with potent enzymatic and cell-based inhibition of LDH enzymatic activity. Lead compounds such as 63 exhibit low nM inhibition of both LDHA and LDHB, submicromolar inhibition of lactate production, and inhibition of glycolysis in MiaPaCa2 pancreatic cancer and A673 sarcoma cells. Moreover, robust target engagement of LDHA by lead compounds was demonstrated using the cellular thermal shift assay (CETSA), and drug–target residence time was determined via SPR. Analysis of these data suggests that drug–target residence time (off-rate) may be an important attribute to consider for obtaining potent cell-based inhibition of this cancer metabolism target.
中文翻译:

乳酸脱氢酶 (LDH) 强效细胞活性吡唑基抑制剂的发现和优化
我们报告了一系列新的基于吡唑的人乳酸脱氢酶 (LDH) 抑制剂的发现和药物化学优化。定量高通量筛选范式的利用促进了命中识别,而基于结构的设计和多参数优化使得能够开发具有有效酶促和基于细胞的 LDH 酶促活性抑制作用的化合物。63等先导化合物在 MiaPaCa2 胰腺癌和 A673 肉瘤细胞中表现出对 LDHA 和 LDHB 的低 nM 抑制、对乳酸产生的亚微摩尔抑制以及对糖酵解的抑制。此外,使用细胞热转移分析 (CETSA) 证明了先导化合物对 LDHA 的稳健目标参与,并通过 SPR 确定了药物-目标停留时间。对这些数据的分析表明,药物-靶标停留时间(解离率)可能是获得对这种癌症代谢靶标的有效细胞抑制时需要考虑的一个重要属性。
更新日期:2017-11-09
中文翻译:

乳酸脱氢酶 (LDH) 强效细胞活性吡唑基抑制剂的发现和优化
我们报告了一系列新的基于吡唑的人乳酸脱氢酶 (LDH) 抑制剂的发现和药物化学优化。定量高通量筛选范式的利用促进了命中识别,而基于结构的设计和多参数优化使得能够开发具有有效酶促和基于细胞的 LDH 酶促活性抑制作用的化合物。63等先导化合物在 MiaPaCa2 胰腺癌和 A673 肉瘤细胞中表现出对 LDHA 和 LDHB 的低 nM 抑制、对乳酸产生的亚微摩尔抑制以及对糖酵解的抑制。此外,使用细胞热转移分析 (CETSA) 证明了先导化合物对 LDHA 的稳健目标参与,并通过 SPR 确定了药物-目标停留时间。对这些数据的分析表明,药物-靶标停留时间(解离率)可能是获得对这种癌症代谢靶标的有效细胞抑制时需要考虑的一个重要属性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号