当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Micelle formation as a factor influencing the mode(s) of metal ion partitioning into N-alkylpyridinium-based ionic liquids (ILs): implications for the design of IL-based extraction systems
Green Chemistry ( IF 9.8 ) Pub Date : 2017-11-09 00:00:00 , DOI: 10.1039/c7gc02338c James L. Wankowski 1, 2, 3, 4 , Michael J. Kaul 1, 2, 3, 4 , Mark L. Dietz 1, 2, 3, 4
Green Chemistry ( IF 9.8 ) Pub Date : 2017-11-09 00:00:00 , DOI: 10.1039/c7gc02338c James L. Wankowski 1, 2, 3, 4 , Michael J. Kaul 1, 2, 3, 4 , Mark L. Dietz 1, 2, 3, 4
Affiliation
|
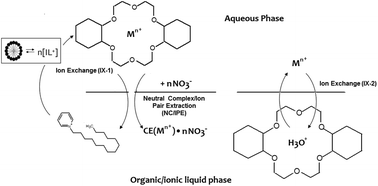
Prior studies of metal ion partitioning between an acidic aqueous phase and an ionic liquid in the presence of a macrocyclic polyether have demonstrated that the overall partitioning is a composite of three distinct pathways: neutral complex/ion-pair extraction, exchange of a cationic metal-crown ether (CE) complex for the cationic constituent of the IL, and exchange of the metal ion for a hydronium ion in a CE-H3O+ complex formed during acid preconditioning of the IL. The obvious undesirability of the ion-exchange pathways, which can lead to substantial loss of the IL cation to the aqueous phase, has led to efforts to identify means by which these processes can be suppressed or eliminated. Prior work with N,N′-dialkylimidazolium and quaternary ammonium bis [(trifluoromethyl)sulfonyl]imides has shown that increasing the hydrophobicity of the IL cation can be an effective means of diminishing the contribution of ion-exchange. Work with the corresponding N-alkylpyridinium ILs, however, indicates that in certain instances, an increase in the hydrophobicity of the IL cation is accompanied by a marked increase in its propensity to self-associate, leading to the formation of micelles in the aqueous phase. The net effect is to diminish or even negate the expected beneficial effect of IL cation hydrophobicity in reducing the contribution of ion exchange to the overall metal ion partitioning process, adversely impacting the “greenness” of extraction processes employing these ILs.
中文翻译:

胶束形成是影响金属离子分配到基于N-烷基吡啶鎓的离子液体(IL)的方式的因素:对基于IL的萃取系统设计的启示
先前在大环聚醚存在下在酸性水相和离子液体之间进行金属离子分配的研究表明,整体分配是三个不同途径的组合:中性络合物/离子对萃取,阳离子金属离子交换IL阳离子成分的冠醚(CE)络合物,以及在IL酸预处理期间形成的CE-H 3 O +络合物中金属离子交换为水合氢离子。离子交换途径的明显不合需要,这可能导致IL阳离子大量流失到水相,导致人们努力寻找可以抑制或消除这些过程的手段。N,N的先前工作'-二烷基咪唑鎓和双[(三氟甲基)磺酰基]季铵盐显示,增加IL阳离子的疏水性可以是减少离子交换作用的有效手段。但是,使用相应的N-烷基吡啶鎓ILs进行的研究表明,在某些情况下,IL阳离子的疏水性增加伴随着其自缔合倾向的显着增加,从而导致在水相中形成胶束。最终效果是减少或什至消除了IL阳离子疏水性的预期有益效果,从而减少了离子交换对整个金属离子分配过程的影响,不利地影响了使用这些IL的提取过程的“绿色度”。
更新日期:2017-11-09
中文翻译:

胶束形成是影响金属离子分配到基于N-烷基吡啶鎓的离子液体(IL)的方式的因素:对基于IL的萃取系统设计的启示
先前在大环聚醚存在下在酸性水相和离子液体之间进行金属离子分配的研究表明,整体分配是三个不同途径的组合:中性络合物/离子对萃取,阳离子金属离子交换IL阳离子成分的冠醚(CE)络合物,以及在IL酸预处理期间形成的CE-H 3 O +络合物中金属离子交换为水合氢离子。离子交换途径的明显不合需要,这可能导致IL阳离子大量流失到水相,导致人们努力寻找可以抑制或消除这些过程的手段。N,N的先前工作'-二烷基咪唑鎓和双[(三氟甲基)磺酰基]季铵盐显示,增加IL阳离子的疏水性可以是减少离子交换作用的有效手段。但是,使用相应的N-烷基吡啶鎓ILs进行的研究表明,在某些情况下,IL阳离子的疏水性增加伴随着其自缔合倾向的显着增加,从而导致在水相中形成胶束。最终效果是减少或什至消除了IL阳离子疏水性的预期有益效果,从而减少了离子交换对整个金属离子分配过程的影响,不利地影响了使用这些IL的提取过程的“绿色度”。



























 京公网安备 11010802027423号
京公网安备 11010802027423号