当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Catalyst-free aerobic oxidation of aldehydes into acids in water under mild conditions
Green Chemistry ( IF 9.8 ) Pub Date : 2017-10-23 00:00:00 , DOI: 10.1039/c7gc02983g Yue Zhang 1, 2, 3, 4 , Yujia Cheng 1, 2, 3, 4 , Huizhuo Cai 1, 2, 3, 4 , Shaopo He 1, 2, 3, 4 , Qiheng Shan 1, 2, 3, 4 , Hongwei Zhao 3, 4, 5, 6, 7 , Yiping Chen 1, 2, 3, 4 , Bo Wang 1, 2, 3, 4, 5
Green Chemistry ( IF 9.8 ) Pub Date : 2017-10-23 00:00:00 , DOI: 10.1039/c7gc02983g Yue Zhang 1, 2, 3, 4 , Yujia Cheng 1, 2, 3, 4 , Huizhuo Cai 1, 2, 3, 4 , Shaopo He 1, 2, 3, 4 , Qiheng Shan 1, 2, 3, 4 , Hongwei Zhao 3, 4, 5, 6, 7 , Yiping Chen 1, 2, 3, 4 , Bo Wang 1, 2, 3, 4, 5
Affiliation
|
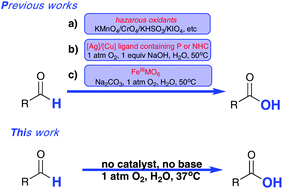
The first example of catalyst-free aerobic oxidation of aldehydes in water under respective acidic, neutral and alkaline conditions was developed. The sole oxidant is molecular oxygen of 1 atmosphere and reactions can proceed under extremely mild conditions. This procedure covers a wide range of aldehydes, and operates easily. No additives and catalysts were required for this purpose, and most of the aldehydes can be converted to their corresponding carboxylic acids with good to excellent yields, in addition, no side-product formation could be observed during or after the reactions. To well illustrate why the oxidation rate becomes fast firstly and then slows with increased temperatures, five control reactions were carried out and a Fe3+/Fe2+ recycling system was introduced to facilitate the aldehyde oxidation rate. The generality of this method offers the potential for industrial aldehyde-containing waste water treatment.
中文翻译:

在温和条件下无催化剂将水中的醛类有氧氧化成酸
开发了在相应的酸性,中性和碱性条件下水中无醛需氧氧化的第一个实例。唯一的氧化剂是1个大气压的分子氧,并且反应可以在极其温和的条件下进行。该程序涵盖了广泛的醛类,并且操作简便。为此不需要添加剂和催化剂,并且大多数醛可以良好或优异的产率转化为它们相应的羧酸,此外,在反应期间或之后没有观察到副产物的形成。为了很好地说明为什么氧化速率会随着温度的升高先变快然后变慢的原因,进行了五个控制反应,然后进行了Fe 3+ / Fe 2+引入了再循环系统以促进醛的氧化速率。这种方法的通用性为工业含醛废水处理提供了潜力。
更新日期:2017-11-09
中文翻译:

在温和条件下无催化剂将水中的醛类有氧氧化成酸
开发了在相应的酸性,中性和碱性条件下水中无醛需氧氧化的第一个实例。唯一的氧化剂是1个大气压的分子氧,并且反应可以在极其温和的条件下进行。该程序涵盖了广泛的醛类,并且操作简便。为此不需要添加剂和催化剂,并且大多数醛可以良好或优异的产率转化为它们相应的羧酸,此外,在反应期间或之后没有观察到副产物的形成。为了很好地说明为什么氧化速率会随着温度的升高先变快然后变慢的原因,进行了五个控制反应,然后进行了Fe 3+ / Fe 2+引入了再循环系统以促进醛的氧化速率。这种方法的通用性为工业含醛废水处理提供了潜力。


























 京公网安备 11010802027423号
京公网安备 11010802027423号