Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ion mobility-resolved collision-induced dissociation and electron transfer dissociation of N-glycopeptides: gathering orthogonal connectivity information from a single mass-selected precursor ion population
Analyst ( IF 4.2 ) Pub Date : 2017-11-03 00:00:00 , DOI: 10.1039/c7an01196b Venkata Kolli 1, 2, 3, 4 , Katherine N. Schumacher 1, 2, 3, 4 , Eric D. Dodds 1, 2, 3, 4
Analyst ( IF 4.2 ) Pub Date : 2017-11-03 00:00:00 , DOI: 10.1039/c7an01196b Venkata Kolli 1, 2, 3, 4 , Katherine N. Schumacher 1, 2, 3, 4 , Eric D. Dodds 1, 2, 3, 4
Affiliation
|
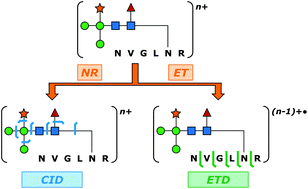
Glycopeptide-level mass spectrometry (MS) and tandem mass spectrometry (MS/MS) analyses are commonly performed to establish site-specific protein glycosylation profiles that are of central importance to gaining structure–function insights on glycoproteins. Confoundingly, the complete characterization of glycopeptide connectivity usually requires the acquisition of multiple MS/MS fragmentation spectra. Complementary ion fragmentation techniques such as collision-induced dissociation (CID) and electron transfer dissociation (ETD) are often applied in concert to address this need. While structurally informative, the requirement for acquisition of two MS/MS spectra per analyte places considerable limitations upon the breadth and depth of large-scale glycoproteomic inquiry. Here, a previously developed method of multiplexing CID and ETD is applied to the study of glycopeptides for the first time. Integration of the two dissociation methods was accomplished through addition of an ion mobility (IM) dimension that disperses the two stages of MS/MS in time. This allows the two MS/MS spectra to be acquired within a few milliseconds of one another, and to be deconvoluted in post-processing. Furthermore, the method allows both fragmentation readouts to be obtained from the same precursor ion packet, thus reducing the inefficiencies imposed by separate CID and ETD acquisitions and the relatively poor precursor ion to fragment ion conversion typical of ETD. N-Linked glycopeptide ions ranging in molecular weight from 1.8 to 6.5 kDa were generated from four model glycoproteins that collectively encompassed paucimannosidic, high mannose, and complex types of N-glycosylation. In each case, IM-resolved CID and ETD events provided complete coverage of the glycan topology and peptide sequence coverages ranging from 48.4% (over 32 amino acid residues) to 85.7% (over eight amino acid residues). The potential of this method for large-scale glycoproteomic analysis is discussed.
中文翻译:

N糖肽的离子淌度分辨碰撞诱导解离和电子转移解离:从单个质量选择的前体离子群体中收集正交连通性信息
糖肽级质谱(MS)和串联质谱(MS / MS)分析通常用于建立位点特异性蛋白糖基化谱,这对于获得糖蛋白的结构-功能见解至关重要。令人困惑的是,糖肽连接性的完整表征通常需要采集多个MS / MS碎片质谱图。互补离子碎裂技术(例如碰撞诱导解离(CID)和电子转移解离(ETD))经常一起使用,以解决这一需求。虽然在结构上提供信息,但每个分析物需要采集两个MS / MS光谱的要求对大规模糖蛋白组学研究的广度和深度提出了相当大的限制。这里,先前开发的将CID和ETD复用的方法首次用于糖肽的研究。两种解离方法的集成是通过添加离子迁移率(IM)维度来完成的,该维度可以及时分散MS / MS的两个阶段。这样一来,两个MS / MS谱图就可以在几毫秒内相互获取,并在后处理中进行去卷积处理。此外,该方法允许从相同的前驱体离子包中获得两个碎片读数,从而减少了由单独的CID和ETD采集以及ETD典型的相对较差的前驱体离子到碎片离子的转换所带来的效率低下。两种解离方法的集成是通过添加离子迁移率(IM)维度来完成的,该维度可以及时分散MS / MS的两个阶段。这样一来,两个MS / MS谱图就可以在几毫秒内相互获取,并在后处理中进行去卷积处理。此外,该方法允许从相同的前驱体离子包中获得两个碎片读数,从而减少了由单独的CID和ETD采集以及ETD典型的相对较差的前驱体离子到碎片离子的转换所带来的效率低下。两种解离方法的集成是通过添加离子迁移率(IM)维度来完成的,该维度可以及时分散MS / MS的两个阶段。这样一来,两个MS / MS谱图就可以在几毫秒内相互获取,并在后处理中进行去卷积处理。此外,该方法允许从相同的前驱体离子包中获得两个碎片读数,从而减少了由单独的CID和ETD采集以及ETD典型的相对较差的前驱体离子到碎片离子的转换所带来的效率低下。从四种模型糖蛋白生成分子量范围为1.8至6.5 kDa的N-连接糖肽离子,这些糖蛋白共同包含古甘露糖苷,高甘露糖和复杂类型的N-糖基化。在每种情况下,IM解析的CID和ETD事件提供了聚糖拓扑结构和肽序列的完整覆盖范围,范围从48.4%(超过32个氨基酸残基)到85.7%(超过8个氨基酸残基)。讨论了该方法在大规模糖蛋白组学分析中的潜力。
更新日期:2017-11-09
中文翻译:

N糖肽的离子淌度分辨碰撞诱导解离和电子转移解离:从单个质量选择的前体离子群体中收集正交连通性信息
糖肽级质谱(MS)和串联质谱(MS / MS)分析通常用于建立位点特异性蛋白糖基化谱,这对于获得糖蛋白的结构-功能见解至关重要。令人困惑的是,糖肽连接性的完整表征通常需要采集多个MS / MS碎片质谱图。互补离子碎裂技术(例如碰撞诱导解离(CID)和电子转移解离(ETD))经常一起使用,以解决这一需求。虽然在结构上提供信息,但每个分析物需要采集两个MS / MS光谱的要求对大规模糖蛋白组学研究的广度和深度提出了相当大的限制。这里,先前开发的将CID和ETD复用的方法首次用于糖肽的研究。两种解离方法的集成是通过添加离子迁移率(IM)维度来完成的,该维度可以及时分散MS / MS的两个阶段。这样一来,两个MS / MS谱图就可以在几毫秒内相互获取,并在后处理中进行去卷积处理。此外,该方法允许从相同的前驱体离子包中获得两个碎片读数,从而减少了由单独的CID和ETD采集以及ETD典型的相对较差的前驱体离子到碎片离子的转换所带来的效率低下。两种解离方法的集成是通过添加离子迁移率(IM)维度来完成的,该维度可以及时分散MS / MS的两个阶段。这样一来,两个MS / MS谱图就可以在几毫秒内相互获取,并在后处理中进行去卷积处理。此外,该方法允许从相同的前驱体离子包中获得两个碎片读数,从而减少了由单独的CID和ETD采集以及ETD典型的相对较差的前驱体离子到碎片离子的转换所带来的效率低下。两种解离方法的集成是通过添加离子迁移率(IM)维度来完成的,该维度可以及时分散MS / MS的两个阶段。这样一来,两个MS / MS谱图就可以在几毫秒内相互获取,并在后处理中进行去卷积处理。此外,该方法允许从相同的前驱体离子包中获得两个碎片读数,从而减少了由单独的CID和ETD采集以及ETD典型的相对较差的前驱体离子到碎片离子的转换所带来的效率低下。从四种模型糖蛋白生成分子量范围为1.8至6.5 kDa的N-连接糖肽离子,这些糖蛋白共同包含古甘露糖苷,高甘露糖和复杂类型的N-糖基化。在每种情况下,IM解析的CID和ETD事件提供了聚糖拓扑结构和肽序列的完整覆盖范围,范围从48.4%(超过32个氨基酸残基)到85.7%(超过8个氨基酸残基)。讨论了该方法在大规模糖蛋白组学分析中的潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号