当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and Optical Properties of Excited-State Intramolecular Proton Transfer Active π-Conjugated Benzimidazole Compounds: Influence of Structural Rigidification by Ring Fusion
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2017-11-08 00:00:00 , DOI: 10.1021/acs.joc.7b01967 Koji Takagi 1 , Kaede Ito 1 , Yoshihiro Yamada 1 , Takuya Nakashima 2 , Ryoichi Fukuda 3, 4 , Masahiro Ehara 3, 4 , Hyuma Masu 5
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2017-11-08 00:00:00 , DOI: 10.1021/acs.joc.7b01967 Koji Takagi 1 , Kaede Ito 1 , Yoshihiro Yamada 1 , Takuya Nakashima 2 , Ryoichi Fukuda 3, 4 , Masahiro Ehara 3, 4 , Hyuma Masu 5
Affiliation
|
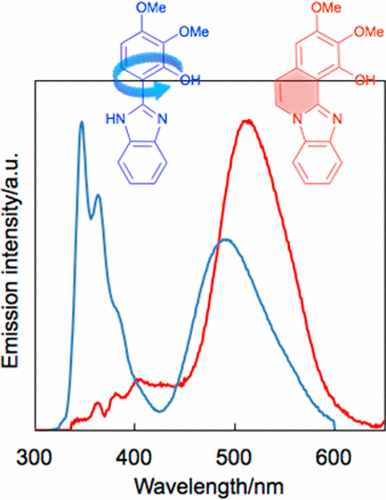
Two excited-state intramolecular proton transfer (ESIPT) active benzimidazole derivatives (1 and 2) were synthesized by acid-catalyzed intramolecular cyclization. The steady-state fluorescence spectrum in THF revealed that ring-fused derivative 1 exhibits a dual emission, namely, the major emission was from the K* (keto) form (ESIPT emission) at 515 nm with a large Stokes shift of 11 100 cm–1 and the minor emission was from the E* (enol) form at below 400 nm. In contrast, the normal emission from the E* form was dominant and the fluorescence quantum yield was very low (Φ ∼ 0.002) for nonfused derivative 2. The time-resolved fluorescence spectroscopy of 1 suggested that ESIPT effectively occurs due to the restricted conformational transition to the S1–TICT state, and the averaged radiative and nonradiative decay rate constants were estimated as ⟨kf⟩ = 0.15 ns–1 and ⟨knr⟩ = 0.60 ns–1, respectively. The fluorescence emission of 1 was influenced by the measurement conditions, such as solvent polarity and basicity, as well as the presence of Lewis base. The ESIPT process and solvatochromic behavior were nicely reproduced by the DFT/TDDFT calculation using the PCM model. In the single-crystal fluorescent spectra, the ESIPT emissions were exclusively observed for both fused and nonfused compounds as a result of hydrogen-bonding interactions.
中文翻译:

激发态分子内质子转移活性π共轭苯并咪唑化合物的合成和光学性质:环融合对结构刚性的影响
通过酸催化的分子内环化反应,合成了两种激发态的分子内质子转移(ESIPT)活性苯并咪唑衍生物(1和2)。THF中的稳态荧光光谱表明,环稠合衍生物1表现出双重发射,即主要发射来自515 nm处的K *(酮基)形式(ESIPT发射),斯托克斯位移为11100 cm –1,次要发射来自低于400 nm的E *(烯醇)形式。相反,非融合衍生物2的E *形式的正态发射占主导,荧光量子产率非常低(Φ〜0.002)。的时间分辨荧光光谱1建议ESIPT有效的发生是由于受限制的构象转变到S 1 -T ICT状态,和平均辐射和非辐射衰变速率常数估计为⟨ ķ ˚F ⟩= 0.15纳秒-1和⟨ ķ NR ⟩= 0.60纳秒-分别为1。1的荧光发射受测量条件的影响,例如溶剂的极性和碱性,以及路易斯碱的存在。通过使用PCM模型进行DFT / TDDFT计算,很好地再现了ESIPT过程和溶剂变色行为。在单晶荧光光谱中,由于氢键相互作用,仅观察到熔融和未熔融化合物的ESIPT发射。
更新日期:2017-11-09
中文翻译:

激发态分子内质子转移活性π共轭苯并咪唑化合物的合成和光学性质:环融合对结构刚性的影响
通过酸催化的分子内环化反应,合成了两种激发态的分子内质子转移(ESIPT)活性苯并咪唑衍生物(1和2)。THF中的稳态荧光光谱表明,环稠合衍生物1表现出双重发射,即主要发射来自515 nm处的K *(酮基)形式(ESIPT发射),斯托克斯位移为11100 cm –1,次要发射来自低于400 nm的E *(烯醇)形式。相反,非融合衍生物2的E *形式的正态发射占主导,荧光量子产率非常低(Φ〜0.002)。的时间分辨荧光光谱1建议ESIPT有效的发生是由于受限制的构象转变到S 1 -T ICT状态,和平均辐射和非辐射衰变速率常数估计为⟨ ķ ˚F ⟩= 0.15纳秒-1和⟨ ķ NR ⟩= 0.60纳秒-分别为1。1的荧光发射受测量条件的影响,例如溶剂的极性和碱性,以及路易斯碱的存在。通过使用PCM模型进行DFT / TDDFT计算,很好地再现了ESIPT过程和溶剂变色行为。在单晶荧光光谱中,由于氢键相互作用,仅观察到熔融和未熔融化合物的ESIPT发射。


























 京公网安备 11010802027423号
京公网安备 11010802027423号