当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Heightened Dynamics of the Oxidized Y48H Variant of Human Cytochrome c Increases Its Peroxidatic Activity
Biochemistry ( IF 2.9 ) Pub Date : 2017-11-08 00:00:00 , DOI: 10.1021/acs.biochem.7b00890 Oliver M. Deacon 1 , Andreas Ioannis Karsisiotis 1 , Tadeo Moreno-Chicano 1 , Michael A. Hough 1 , Colin Macdonald 2 , Tharin M. A. Blumenschein 2 , Michael T. Wilson 1 , Geoffrey R. Moore 2 , Jonathan A. R. Worrall 1
Biochemistry ( IF 2.9 ) Pub Date : 2017-11-08 00:00:00 , DOI: 10.1021/acs.biochem.7b00890 Oliver M. Deacon 1 , Andreas Ioannis Karsisiotis 1 , Tadeo Moreno-Chicano 1 , Michael A. Hough 1 , Colin Macdonald 2 , Tharin M. A. Blumenschein 2 , Michael T. Wilson 1 , Geoffrey R. Moore 2 , Jonathan A. R. Worrall 1
Affiliation
|
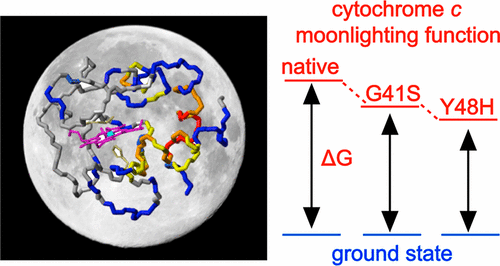
Proteins performing multiple biochemical functions are called “moonlighting proteins” or extreme multifunctional (EMF) proteins. Mitochondrial cytochrome c is an EMF protein that binds multiple partner proteins to act as a signaling molecule, transfers electrons in the respiratory chain, and acts as a peroxidase in apoptosis. Mutations in the cytochrome c gene lead to the disease thrombocytopenia, which is accompanied by enhanced apoptotic activity. The Y48H variant arises from one such mutation and is found in the 40–57 Ω-loop, the lowest-unfolding free energy substructure of the cytochrome c fold. A 1.36 Å resolution X-ray structure of the Y48H variant reveals minimal structural changes compared to the wild-type structure, with the axial Met80 ligand coordinated to the heme iron. Despite this, the intrinsic peroxidase activity is enhanced, implying that a pentacoordinate heme state is more prevalent in the Y48H variant, corroborated through determination of a Met80 “off rate” of >125 s–1 compared to a rate of ∼6 s–1 for the wild-type protein. Heteronuclear nuclear magnetic resonance measurements with the oxidized Y48H variant reveal heightened dynamics in the 40–57 Ω-loop and the Met80-containing 71–85 Ω-loop relative to the wild-type protein, illustrating communication between these substructures. Placed into context with the G41S cytochrome c variant, also implicated in thrombocytopenia, a dynamic picture associated with this disease relative to cytochrome c is emerging whereby increasing dynamics in substructures of the cytochrome c fold serve to facilitate an increased population of the peroxidatic pentacoordinate heme state in the following order: wild type < G41S < Y48H.
中文翻译:

人类细胞色素c的氧化Y48H变体的动力学增强增加了其过氧化活性。
具有多种生化功能的蛋白质称为“月光蛋白质”或极端多功能(EMF)蛋白质。线粒体细胞色素c是一种EMF蛋白,可结合多个伴侣蛋白以充当信号分子,在呼吸链中转移电子,并在细胞凋亡中充当过氧化物酶。细胞色素c基因的突变会导致血小板减少症,并伴有凋亡活性的增强。Y48H变体源自一种这样的突变,发现于40-57Ω环中,这是细胞色素c的最低展开自由能亚结构折叠。与野生型结构相比,Y48H变体的1.36Å分辨率X射线结构显示出最小的结构变化,轴向Met80配体与血红素铁配位。尽管如此,固有的过氧化物酶活性仍得到增强,这意味着在Y48H变体中五配位血红素状态更为普遍,这通过确定Met80的“关闭速率”> 125 s –1相比于〜6 s –1的速率得到了证实。为野生型蛋白质。氧化的Y48H变体的异核核磁共振测量表明,相对于野生型蛋白质,其40–57Ω环和含有Met80的71–85Ω环的动力学增强,说明了这些亚结构之间的通讯。与G41S细胞色素c结合使用变体,也与血小板减少有关,这种疾病相对于细胞色素c的动态图片正在出现,由此细胞色素c折叠亚结构的动态增加有助于按以下顺序增加过氧化物五配位血红素状态的种群:野生型< G41S <Y48H。
更新日期:2017-11-09
中文翻译:

人类细胞色素c的氧化Y48H变体的动力学增强增加了其过氧化活性。
具有多种生化功能的蛋白质称为“月光蛋白质”或极端多功能(EMF)蛋白质。线粒体细胞色素c是一种EMF蛋白,可结合多个伴侣蛋白以充当信号分子,在呼吸链中转移电子,并在细胞凋亡中充当过氧化物酶。细胞色素c基因的突变会导致血小板减少症,并伴有凋亡活性的增强。Y48H变体源自一种这样的突变,发现于40-57Ω环中,这是细胞色素c的最低展开自由能亚结构折叠。与野生型结构相比,Y48H变体的1.36Å分辨率X射线结构显示出最小的结构变化,轴向Met80配体与血红素铁配位。尽管如此,固有的过氧化物酶活性仍得到增强,这意味着在Y48H变体中五配位血红素状态更为普遍,这通过确定Met80的“关闭速率”> 125 s –1相比于〜6 s –1的速率得到了证实。为野生型蛋白质。氧化的Y48H变体的异核核磁共振测量表明,相对于野生型蛋白质,其40–57Ω环和含有Met80的71–85Ω环的动力学增强,说明了这些亚结构之间的通讯。与G41S细胞色素c结合使用变体,也与血小板减少有关,这种疾病相对于细胞色素c的动态图片正在出现,由此细胞色素c折叠亚结构的动态增加有助于按以下顺序增加过氧化物五配位血红素状态的种群:野生型< G41S <Y48H。



























 京公网安备 11010802027423号
京公网安备 11010802027423号