当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Fluorescamine Labeling for Assessment of Protein Conformational Change and Binding Affinity in Protein–Nanoparticle Interaction
Analytical Chemistry ( IF 7.4 ) Pub Date : 2017-11-08 00:00:00 , DOI: 10.1021/acs.analchem.7b02810 Yaokai Duan 1 , Yang Liu 1 , Wen Shen 1 , Wenwan Zhong 1
Analytical Chemistry ( IF 7.4 ) Pub Date : 2017-11-08 00:00:00 , DOI: 10.1021/acs.analchem.7b02810 Yaokai Duan 1 , Yang Liu 1 , Wen Shen 1 , Wenwan Zhong 1
Affiliation
|
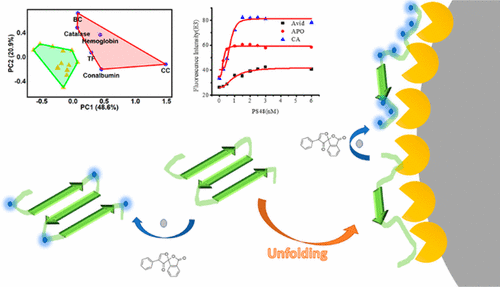
Protein adsorption alters the “biological identity” of nanoparticles (NPs) and could affect how biosystems respond to invading NPs. Study of protein–NP interaction can help understand how the physicochemical properties of NPs impact the interaction and thus potentially guide the design of safer and more effective NPs for biomedical or other applications. Binding affinity between proteins and NPs and the occurrence of protein conformational change upon binding to NPs are two important aspects to be learned, but few methods are currently available to assess both simultaneously in a simple way. Herein, we demonstrated that the fluorescamine labeling method developed by our group not only could reveal protein conformational change upon adsorption to NPs, owing to its capability to label the primary amines exposed on protein surface, but also could be applied to measure the binding affinity. By screening the interaction between a large number of proteins and four types of NPs, the present study also revealed that protein adsorption onto NPs could be strongly affected by structure flexibility. The proteins with high structure flexibility experienced high degrees of conformation change when binding to the polystyrene NPs, which could potentially influence protein function. Overall, we demonstrate that our assay is a quick, simple, and high-throughput tool to reveal potential impacts on protein activity and evaluate the strength of protein–NP binding.
中文翻译:

荧光胺标记,用于评估蛋白质-纳米粒子相互作用中的蛋白质构象变化和结合亲和力
蛋白质吸附会改变纳米颗粒(NP)的“生物学特性”,并可能影响生物系统对入侵NP的反应。蛋白质-NP相互作用的研究可以帮助理解NP的理化特性如何影响相互作用,从而有可能指导生物医学或其他应用中更安全,更有效的NP的设计。蛋白质和NP之间的结合亲和力以及与NP结合后蛋白质构象变化的发生是需要学习的两个重要方面,但是目前很少有方法可以通过简单的方式同时评估两者。在这里,我们证明了我们小组开发的荧光胺标记方法不仅可以揭示吸附到NP上的蛋白质构象变化,还因为它能够标记暴露在蛋白质表面的伯胺,但也可用于测量结合亲和力。通过筛选大量蛋白质与四种类型的NP之间的相互作用,本研究还揭示了蛋白质吸附到NP上可能会受到结构柔韧性的强烈影响。具有高结构柔性的蛋白质在与聚苯乙烯NP结合时会经历高度的构象变化,这可能会影响蛋白质的功能。总体而言,我们证明了我们的测定方法是一种快速,简单且高通量的工具,可揭示对蛋白质活性的潜在影响并评估蛋白质与NP的结合强度。本研究还表明,蛋白质吸附到NP上可能会受到结构柔韧性的强烈影响。具有高结构柔性的蛋白质在与聚苯乙烯NP结合时会经历高度的构象变化,这可能会影响蛋白质的功能。总体而言,我们证明了我们的测定方法是一种快速,简单且高通量的工具,可揭示对蛋白质活性的潜在影响并评估蛋白质与NP的结合强度。本研究还表明,蛋白质吸附到NP上可能会受到结构柔韧性的强烈影响。具有高结构柔性的蛋白质在与聚苯乙烯NP结合时会经历高度的构象变化,这可能会影响蛋白质的功能。总体而言,我们证明了我们的测定方法是一种快速,简单且高通量的工具,可揭示对蛋白质活性的潜在影响并评估蛋白质与NP的结合强度。
更新日期:2017-11-09
中文翻译:

荧光胺标记,用于评估蛋白质-纳米粒子相互作用中的蛋白质构象变化和结合亲和力
蛋白质吸附会改变纳米颗粒(NP)的“生物学特性”,并可能影响生物系统对入侵NP的反应。蛋白质-NP相互作用的研究可以帮助理解NP的理化特性如何影响相互作用,从而有可能指导生物医学或其他应用中更安全,更有效的NP的设计。蛋白质和NP之间的结合亲和力以及与NP结合后蛋白质构象变化的发生是需要学习的两个重要方面,但是目前很少有方法可以通过简单的方式同时评估两者。在这里,我们证明了我们小组开发的荧光胺标记方法不仅可以揭示吸附到NP上的蛋白质构象变化,还因为它能够标记暴露在蛋白质表面的伯胺,但也可用于测量结合亲和力。通过筛选大量蛋白质与四种类型的NP之间的相互作用,本研究还揭示了蛋白质吸附到NP上可能会受到结构柔韧性的强烈影响。具有高结构柔性的蛋白质在与聚苯乙烯NP结合时会经历高度的构象变化,这可能会影响蛋白质的功能。总体而言,我们证明了我们的测定方法是一种快速,简单且高通量的工具,可揭示对蛋白质活性的潜在影响并评估蛋白质与NP的结合强度。本研究还表明,蛋白质吸附到NP上可能会受到结构柔韧性的强烈影响。具有高结构柔性的蛋白质在与聚苯乙烯NP结合时会经历高度的构象变化,这可能会影响蛋白质的功能。总体而言,我们证明了我们的测定方法是一种快速,简单且高通量的工具,可揭示对蛋白质活性的潜在影响并评估蛋白质与NP的结合强度。本研究还表明,蛋白质吸附到NP上可能会受到结构柔韧性的强烈影响。具有高结构柔性的蛋白质在与聚苯乙烯NP结合时会经历高度的构象变化,这可能会影响蛋白质的功能。总体而言,我们证明了我们的测定方法是一种快速,简单且高通量的工具,可揭示对蛋白质活性的潜在影响并评估蛋白质与NP的结合强度。


























 京公网安备 11010802027423号
京公网安备 11010802027423号