当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A rapid construction of a specific quino[4,3-b] carbazolone system and its application for the synthesis of calothrixin B
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2017-11-02 00:00:00 , DOI: 10.1039/c7qo00864c Kai Lin 1, 2, 3, 4, 5 , Yong Jian 4, 5, 6, 7 , Peng Zhao 6, 7, 8, 9 , Chun-shen Zhao 6, 7, 8, 9 , Wei-dong Pan 4, 5, 6, 7 , Sheng Liu 4, 5, 6, 7
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2017-11-02 00:00:00 , DOI: 10.1039/c7qo00864c Kai Lin 1, 2, 3, 4, 5 , Yong Jian 4, 5, 6, 7 , Peng Zhao 6, 7, 8, 9 , Chun-shen Zhao 6, 7, 8, 9 , Wei-dong Pan 4, 5, 6, 7 , Sheng Liu 4, 5, 6, 7
Affiliation
|
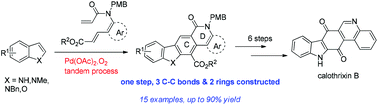
An efficient methodology is developed for the synthesis of functionalized specific carbazole lactams related to pyrido[4,3-b]- and quino[4,3-b] carbazole alkaloids. 3 C–C bonds and 2 rings are created in a single step via an indole-to-carbazole transformation triggered by Pd(II)-catalyzed direct C-3 alkenylation of indoles. Starting from a key pentacyclic precursor delivered by the methodology, calothrixin B is synthesized in 6 steps.
中文翻译:

一种特定的喹[4,3- b ]咔唑啉酮体系的快速构建及其在合成calothrixin B中的应用
开发了一种有效的方法,用于合成与吡啶并[4,3- b ]-和喹[4,3- b ]咔唑生物碱相关的功能化特定咔唑内酰胺。3 C-C键和2个环是在单个步骤中创建经由通过将Pd(触发的吲哚向咔唑转化II)催化的吲哚的直接C-3烯基。从通过该方法提供的关键五环前体开始,Calothrixin B分6个步骤合成。
更新日期:2017-11-08
中文翻译:

一种特定的喹[4,3- b ]咔唑啉酮体系的快速构建及其在合成calothrixin B中的应用
开发了一种有效的方法,用于合成与吡啶并[4,3- b ]-和喹[4,3- b ]咔唑生物碱相关的功能化特定咔唑内酰胺。3 C-C键和2个环是在单个步骤中创建经由通过将Pd(触发的吲哚向咔唑转化II)催化的吲哚的直接C-3烯基。从通过该方法提供的关键五环前体开始,Calothrixin B分6个步骤合成。


























 京公网安备 11010802027423号
京公网安备 11010802027423号