当前位置:
X-MOL 学术
›
Inorg. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
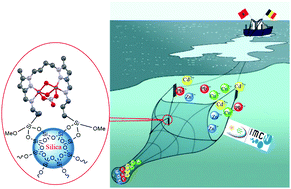
A novel environment-friendly hybrid material based on a modified silica gel with a bispyrazole derivative for the removal of ZnII, PbII, CdII and CuII traces from aqueous solutions
Inorganic Chemistry Frontiers ( IF 7 ) Pub Date : 2017-09-14 00:00:00 , DOI: 10.1039/c7qi00322f Smaail Radi 1, 2, 3, 4, 5 , Mohamed El Massaoudi 1, 2, 3, 4, 5 , Maryse Bacquet 6, 7, 8, 9, 10 , Stéphanie Degoutin 6, 7, 8, 9, 10 , N. N. Adarsh 11, 12, 13, 14, 15 , Koen Robeyns 11, 12, 13, 14, 15 , Yann Garcia 11, 12, 13, 14, 15
Inorganic Chemistry Frontiers ( IF 7 ) Pub Date : 2017-09-14 00:00:00 , DOI: 10.1039/c7qi00322f Smaail Radi 1, 2, 3, 4, 5 , Mohamed El Massaoudi 1, 2, 3, 4, 5 , Maryse Bacquet 6, 7, 8, 9, 10 , Stéphanie Degoutin 6, 7, 8, 9, 10 , N. N. Adarsh 11, 12, 13, 14, 15 , Koen Robeyns 11, 12, 13, 14, 15 , Yann Garcia 11, 12, 13, 14, 15
Affiliation
|
The extraction and separation of heavy metals with several hybrid sorbents has been recognized as a promising green methodology. We present a novel and efficient host based on a modified silica gel with a bis(pyrazole)butane derivative as a chelating ligand; it was synthesized and studied for its adsorption capacity towards toxic heavy metals in aqueous media. The success of the synthesis of SiNAL4 was concluded from elemental analysis, Fourier transform-infrared spectroscopy, solid state 13C NMR, nitrogen adsorption–desorption isotherm, BET surface area, BJH pore size, scanning electron microscopy and thermogravimetry analysis. The effectiveness, regenerability and stability of the studied adsorbent were demonstrated. The optimum conditions for the removal of selected toxic metal ions were found to be pH = 6 and 25 min of contact time with the hybrid material. The maximum levels of ZnII, PbII, CdII and CuII uptake by the hybrid material were 86.51, 35.26, 26.96 and 20.24 mg g−1, respectively. The equilibrium isotherm models of the adsorbent were better described by a Langmuir model. The kinetics of heavy metal adsorption of the hybrid material followed a pseudo-second-order model and the thermodynamic parameters confirmed the spontaneous adsorption, and the spontaneity increases with the increase in temperature. The architecture of the hybrid material, when adsorbing the metal on the surface of the inorganic silica, was revealed using single crystal X-ray diffraction of a model material capturing CuII ions thanks to ligand coordination. Finally, the hybrid material was used in the treatment of river waters containing ZnII and CdII. The quantitative removal of the toxic metals under study from real aqueous solutions confirms the effectiveness of this new adsorbent compared to several previously described materials.
中文翻译:

基于改性硅胶和双吡唑衍生物的新型环保杂化材料,用于从水溶液中去除痕量的Zn II,Pb II,Cd II和Cu II
用几种混合吸附剂萃取和分离重金属已被认为是一种有前途的绿色方法。我们提出了一种基于双(吡唑)丁烷衍生物作为螯合配体的改性硅胶的新型高效宿主。合成并研究了其在水性介质中对有毒重金属的吸附能力。通过元素分析,傅立叶变换红外光谱,固态13得出了合成SiNAL4的成功信息。13 C NMR,氮吸附-解吸等温线,BET表面积,BJH孔径,扫描电子显微镜和热重分析。证明了所研究吸附剂的有效性,可再生性和稳定性。发现去除选定的有毒金属离子的最佳条件是pH = 6,并且与杂化材料的接触时间为25分钟。杂化材料对Zn II,Pb II,Cd II和Cu II的最大吸收量为86.51、35.26、26.96和20.24 mg g -1, 分别。Langmuir模型可以更好地描述吸附剂的平衡等温线模型。重金属吸附杂化材料的动力学遵循伪二级模型,热力学参数证实了自发吸附,并且自发性随温度的升高而增加。当将金属吸附在无机二氧化硅表面上时,由于配体配位,使用捕获Cu II离子的模型材料的单晶X射线衍射揭示了杂化材料的结构。最后,将杂化材料用于处理含Zn II和Cd II的河水。与几种先前描述的材料相比,从实际水溶液中定量去除正在研究的有毒金属证实了这种新型吸附剂的有效性。
更新日期:2017-11-08
中文翻译:

基于改性硅胶和双吡唑衍生物的新型环保杂化材料,用于从水溶液中去除痕量的Zn II,Pb II,Cd II和Cu II
用几种混合吸附剂萃取和分离重金属已被认为是一种有前途的绿色方法。我们提出了一种基于双(吡唑)丁烷衍生物作为螯合配体的改性硅胶的新型高效宿主。合成并研究了其在水性介质中对有毒重金属的吸附能力。通过元素分析,傅立叶变换红外光谱,固态13得出了合成SiNAL4的成功信息。13 C NMR,氮吸附-解吸等温线,BET表面积,BJH孔径,扫描电子显微镜和热重分析。证明了所研究吸附剂的有效性,可再生性和稳定性。发现去除选定的有毒金属离子的最佳条件是pH = 6,并且与杂化材料的接触时间为25分钟。杂化材料对Zn II,Pb II,Cd II和Cu II的最大吸收量为86.51、35.26、26.96和20.24 mg g -1, 分别。Langmuir模型可以更好地描述吸附剂的平衡等温线模型。重金属吸附杂化材料的动力学遵循伪二级模型,热力学参数证实了自发吸附,并且自发性随温度的升高而增加。当将金属吸附在无机二氧化硅表面上时,由于配体配位,使用捕获Cu II离子的模型材料的单晶X射线衍射揭示了杂化材料的结构。最后,将杂化材料用于处理含Zn II和Cd II的河水。与几种先前描述的材料相比,从实际水溶液中定量去除正在研究的有毒金属证实了这种新型吸附剂的有效性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号