当前位置:
X-MOL 学术
›
Metallomics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Modulation of XPC peptide on binding Tb3+ to Euplotes octocarinatus centrin
Metallomics ( IF 3.4 ) Pub Date : 2017-10-25 00:00:00 , DOI: 10.1039/c7mt00263g Enxian Shi 1, 2, 3, 4, 5 , Wenlong Zhang 1, 2, 3, 4, 5 , Yaqin Zhao 1, 2, 3, 4, 5 , Binsheng Yang 1, 2, 3, 4, 5
Metallomics ( IF 3.4 ) Pub Date : 2017-10-25 00:00:00 , DOI: 10.1039/c7mt00263g Enxian Shi 1, 2, 3, 4, 5 , Wenlong Zhang 1, 2, 3, 4, 5 , Yaqin Zhao 1, 2, 3, 4, 5 , Binsheng Yang 1, 2, 3, 4, 5
Affiliation
|
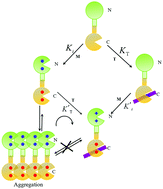
Centrins are Ca2+-binding proteins found throughout eukaryotic organisms. Xeroderma pigmentosum group C protein (XPC), a dominant component of the nuclear excision repair (NER) pathway, is a critical target protein of centrins. A 22-residue peptide (K842-R863) from XPC was used to investigate the effect of metal ions (Ca2+ and Tb3+) on the peptide binding of Euplotes octocarinatus centrin (EoCen) by isothermal titration calorimetry (ITC) and fluorescence spectroscopy. ITC and tryptophan spectrofluorimetric titrations revealed that metal ions (Ca2+ and Tb3+) could enhance the affinity between EoCen and the XPC peptide, and the enhanced effects were closely related to the ion potential of metal ions. Since the ion potential of Tb3+ (e/r = 0.0325) is larger than that of Ca2+ (e/r = 0.0202), the conformational change in the protein induced by Tb3+ is larger than that induced by Ca2+, and the enhanced affinity of Tb3+ is stronger than that of Ca2+. This interaction was driven by enthalpy in the presence of EDTA and enthalpy and entropy in the presence of Ca2+ or Tb3+. Similar to that observed in the presence of EDTA, the N-terminal domain did not participate in the interaction with the XPC peptide even in the presence of metal ions. Resonance light scattering (RLS) and the band shift in native polyacrylamide gel electrophoresis (PAGE) suggested that peptide binding resulted in the dissociation of EoCen aggregates and complex formation via the monomer-peptide form. Tb3+-Sensitized emission suggested that peptide binding in turn also had an impact on the Tb3+ binding of the protein: the C-terminal domain was slightly strengthened and the N-terminal domain was weakened about 225 fold. RLS and native PAGE indicated that the self-assembly induced by Tb3+ binding to the N-terminal domain of EoCen was inhibited in the presence of the XPC peptide. This study elucidates the molecular mechanism of EoCen function in the cellular context.
中文翻译:

XPC肽对Tb 3+与八倍体Euplotes octocarinatus centrin结合的调节
中心蛋白是在整个真核生物中发现的Ca 2+结合蛋白。色素干性干燥菌C组蛋白(XPC)是核切除修复(NER)途径的主要组成部分,是中心蛋白的重要靶蛋白。从XPC一个22残基的肽(K842-R863)被用来研究金属离子(Ca的效果2+和Tb 3+)对肽的结合游仆虫通过等温滴定量热法(ITC)和荧光中心蛋白(EoCen)光谱学。ITC和色氨酸荧光分光光度滴定表明,金属离子(Ca 2+和Tb 3+)可以增强EoCen与XPC肽之间的亲和力,并且增强效果与金属离子的离子电势密切相关。因为Tb的离子电位3+(Ë / [R = 0.0325)比的Ca较大2+(Ë / [R = 0.0202),在用Tb引起的蛋白质的构象变化3+比由钙诱导的较大2 +,并且Tb 3+的亲和力增强,比Ca 2+的亲和力强。这种相互作用是由EDTA存在时的焓和Ca 2+或Tb 3+存在时的焓和熵驱动的。。与在EDTA存在下观察到的相似,即使在存在金属离子的情况下,N末端结构域也不参与与XPC肽的相互作用。共振光散射(RLS)和天然聚丙烯酰胺凝胶电泳(PAGE)中的带移表明,肽结合导致EoCen聚集体解离,并通过单体-肽形式形成复合物。Tb 3+-敏化发射表明,肽结合反过来也对蛋白质的Tb 3+结合也有影响:C末端结构域略微增强,N末端结构域减弱约225倍。RLS和天然PAGE表明Tb 3+引起的自组装在XPC肽存在下,与EoCen N末端结构域的结合被抑制。这项研究阐明了细胞环境中EoCen功能的分子机制。
更新日期:2017-11-08
中文翻译:

XPC肽对Tb 3+与八倍体Euplotes octocarinatus centrin结合的调节
中心蛋白是在整个真核生物中发现的Ca 2+结合蛋白。色素干性干燥菌C组蛋白(XPC)是核切除修复(NER)途径的主要组成部分,是中心蛋白的重要靶蛋白。从XPC一个22残基的肽(K842-R863)被用来研究金属离子(Ca的效果2+和Tb 3+)对肽的结合游仆虫通过等温滴定量热法(ITC)和荧光中心蛋白(EoCen)光谱学。ITC和色氨酸荧光分光光度滴定表明,金属离子(Ca 2+和Tb 3+)可以增强EoCen与XPC肽之间的亲和力,并且增强效果与金属离子的离子电势密切相关。因为Tb的离子电位3+(Ë / [R = 0.0325)比的Ca较大2+(Ë / [R = 0.0202),在用Tb引起的蛋白质的构象变化3+比由钙诱导的较大2 +,并且Tb 3+的亲和力增强,比Ca 2+的亲和力强。这种相互作用是由EDTA存在时的焓和Ca 2+或Tb 3+存在时的焓和熵驱动的。。与在EDTA存在下观察到的相似,即使在存在金属离子的情况下,N末端结构域也不参与与XPC肽的相互作用。共振光散射(RLS)和天然聚丙烯酰胺凝胶电泳(PAGE)中的带移表明,肽结合导致EoCen聚集体解离,并通过单体-肽形式形成复合物。Tb 3+-敏化发射表明,肽结合反过来也对蛋白质的Tb 3+结合也有影响:C末端结构域略微增强,N末端结构域减弱约225倍。RLS和天然PAGE表明Tb 3+引起的自组装在XPC肽存在下,与EoCen N末端结构域的结合被抑制。这项研究阐明了细胞环境中EoCen功能的分子机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号