当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Micellar Catalysis Strategy for Suzuki–Miyaura Cross-Couplings of 2-Pyridyl MIDA Boronates: No Copper, in Water, Very Mild Conditions
ACS Catalysis ( IF 12.9 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acscatal.7b03241 Nicholas A. Isley 1 , Ye Wang 1 , Fabrice Gallou 2 , Sachin Handa 1 , Donald H. Aue 1 , Bruce H. Lipshutz 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acscatal.7b03241 Nicholas A. Isley 1 , Ye Wang 1 , Fabrice Gallou 2 , Sachin Handa 1 , Donald H. Aue 1 , Bruce H. Lipshutz 1
Affiliation
|
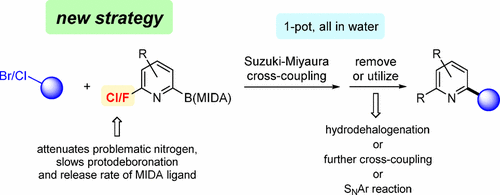
Suzuki–Miyaura (SM) cross-couplings of 2-pyridyl MIDA boronates can be successfully carried out in the complete absence of copper by attenuation of the Lewis basicity associated with the pyridyl nitrogen using selected substituents (e.g., fluorine or chlorine) on the ring. This strategy imparts additional synthetic options compared with existing approaches based on the use of Lewis acids or N-oxides. Thus, access to highly valued 2-substituted pyridyl rings via an initial Suzuki–Miyaura coupling can be followed by dehalogenation, SNAr reactions, or a second SM coupling to arrive at 2,6-disubstituted pyridyl arrays, all run in a single pot, enabled by micellar catalysis in water. Accessing targets within drug-like space is demonstrated in a four-step, one-pot sequence. Computational data suggest that the major role being played by electron-withdrawing substituents in promoting these cross-couplings without the need for copper is to slow the rates of protodeboronation of intermediate 2-pyridylboronic acids.
中文翻译:

Suzuki-Miyaura交联的2-吡啶基MIDA硼酸酯的胶束催化策略:在水中,非常温和的条件下,无铜
通过在环上使用选定的取代基(例如,氟或氯)减弱与吡啶基氮相关的路易斯碱性,可以在完全不存在铜的情况下成功完成2-吡啶基MIDA硼酸酯的Suzuki-Miyaura(SM)交叉偶联。 。与基于路易斯酸或N-氧化物的现有方法相比,该策略赋予了更多的合成选择。因此,通过最初的Suzuki-Miyaura偶联反应可得到高价值的2-取代的吡啶基环,之后可进行脱卤S NAr反应或第二个SM偶联反应可达到2,6-二取代的吡啶基阵列,所有反应均在一个罐中进行,这要归功于在水中的胶束催化。分四步,一锅的顺序演示了在类药物空间内进入靶标的过程。计算数据表明,在不需要铜的情况下,吸电子取代基在促进这些交叉偶联中所起的主要作用是减慢中间体2-吡啶基硼酸的原脱硼硼酸速度。
更新日期:2017-11-07
中文翻译:

Suzuki-Miyaura交联的2-吡啶基MIDA硼酸酯的胶束催化策略:在水中,非常温和的条件下,无铜
通过在环上使用选定的取代基(例如,氟或氯)减弱与吡啶基氮相关的路易斯碱性,可以在完全不存在铜的情况下成功完成2-吡啶基MIDA硼酸酯的Suzuki-Miyaura(SM)交叉偶联。 。与基于路易斯酸或N-氧化物的现有方法相比,该策略赋予了更多的合成选择。因此,通过最初的Suzuki-Miyaura偶联反应可得到高价值的2-取代的吡啶基环,之后可进行脱卤S NAr反应或第二个SM偶联反应可达到2,6-二取代的吡啶基阵列,所有反应均在一个罐中进行,这要归功于在水中的胶束催化。分四步,一锅的顺序演示了在类药物空间内进入靶标的过程。计算数据表明,在不需要铜的情况下,吸电子取代基在促进这些交叉偶联中所起的主要作用是减慢中间体2-吡啶基硼酸的原脱硼硼酸速度。



























 京公网安备 11010802027423号
京公网安备 11010802027423号