当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Two-Silicon Cycle for Carbonyl Hydrosilylation with Nikonov’s Cationic Ruthenium(II) Catalyst
ACS Catalysis ( IF 12.9 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acscatal.7b03336 Julien Fuchs 1 , Hendrik F. T. Klare 1 , Martin Oestreich 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acscatal.7b03336 Julien Fuchs 1 , Hendrik F. T. Klare 1 , Martin Oestreich 1
Affiliation
|
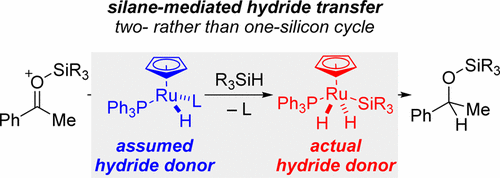
An experimental analysis proves that Nikonov’s carbonyl hydrosilylation proceeds through a two-silicon cycle rather than the originally proposed one-silicon cycle. The intermediate ruthenium(II) monohydride is not sufficiently hydridic to transfer its hydride onto the silylcarboxonium ion. However, that hydricity is enhanced by oxidative addition of another hydrosilane molecule to afford the corresponding ruthenium(IV) silyl dihydride as the actual hydride donor. The present study also demonstrates that the acetonitrile ligands in Nikonov’s ruthenium(II) catalyst are not innocent. That complex is able to hydrosilylate its own ligand(s), and the resulting N,N-disilylated amine base accounts for competing dehydrogenative silylation of enolizable carbonyl compounds, explaining the formation of a silyl enol ether in substantial quantities next to the expected silyl ether. Both findings lead to a revised mechanistic picture that provides the basis for the development of more efficient and chemoselective catalysts.
中文翻译:

尼康诺夫阳离子钌(II)催化剂进行羰基硅氢化的双硅循环
实验分析证明,尼康诺夫的羰基硅氢加成反应是通过两硅循环而不是最初提出的单硅循环进行的。中间体一氢化钌(II)的氢化作用不足,无法将其氢化物转移到甲硅烷基碳鎓离子上。然而,通过氧化添加另一种氢硅烷分子以提供相应的钌(IV)甲硅烷基二氢化物作为实际的氢化物供体,可以提高该水合度。本研究还表明,尼康诺夫钌(II)催化剂中的乙腈配体不是无害的。该配合物能够水解其自身的配体,并生成N,N-二甲硅烷基化的胺碱是可烯醇化的羰基化合物竞争性脱氢甲硅烷基化的原因,解释了与预期的甲硅烷基醚相比,大量甲硅烷基烯醇醚的形成。这两个发现导致了修订后的机理图,为开发更高效和化学选择性的催化剂提供了基础。
更新日期:2017-11-08
中文翻译:

尼康诺夫阳离子钌(II)催化剂进行羰基硅氢化的双硅循环
实验分析证明,尼康诺夫的羰基硅氢加成反应是通过两硅循环而不是最初提出的单硅循环进行的。中间体一氢化钌(II)的氢化作用不足,无法将其氢化物转移到甲硅烷基碳鎓离子上。然而,通过氧化添加另一种氢硅烷分子以提供相应的钌(IV)甲硅烷基二氢化物作为实际的氢化物供体,可以提高该水合度。本研究还表明,尼康诺夫钌(II)催化剂中的乙腈配体不是无害的。该配合物能够水解其自身的配体,并生成N,N-二甲硅烷基化的胺碱是可烯醇化的羰基化合物竞争性脱氢甲硅烷基化的原因,解释了与预期的甲硅烷基醚相比,大量甲硅烷基烯醇醚的形成。这两个发现导致了修订后的机理图,为开发更高效和化学选择性的催化剂提供了基础。



























 京公网安备 11010802027423号
京公网安备 11010802027423号