当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Photo-oxidation of Dimethyl Malonate Initiated by Chlorine Atoms in Gas Phase: Kinetics and Mechanism
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2017-11-08 00:00:00 , DOI: 10.1021/acs.jpca.7b06595 Guido N. Rimondino 1 , Diana P. Henao 1 , Walter J. Peláez 1 , Gustavo A. Argüello 1 , Fabio E. Malanca 1
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2017-11-08 00:00:00 , DOI: 10.1021/acs.jpca.7b06595 Guido N. Rimondino 1 , Diana P. Henao 1 , Walter J. Peláez 1 , Gustavo A. Argüello 1 , Fabio E. Malanca 1
Affiliation
|
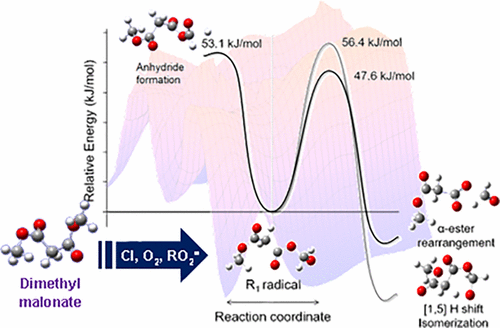
The rate coefficient for the gas-phase reaction of chlorine atoms with dimethyl malonate (DMM, CH3OC(O)CH2C(O)OCH3) was determined at 298 K using relative methods giving a value of (3.8 ± 0.4) × 10–12, cm3 molecule–1 s–1). The photo-oxidation mechanism of DMM was also investigated. The main products were identified by infrared spectroscopy, and computational calculations were performed in order to support the experimental data. The results reveal that the photo-oxidation occurs mainly by the abstraction of an H atom from the methyl groups. The CH3OC(O)CH2C(O)OCH2O• radical formed subsequently reacts according to three competitive paths: reaction with molecular oxygen to yield CH3OC(O)CH2C(O)OC(O)H, isomerization–unimolecular decomposition to lead finally to CH3OC(O)C(O)H, CO2, and HC(O)OH, and α-ester rearrangement to form monomethyl malonate and carbon monoxide. The yield of products as a function of oxygen pressure was also determined.
中文翻译:

气相中氯原子引发的丙二酸二甲酯的光氧化反应:动力学和机理
使用相关方法在298 K下确定氯原子与丙二酸二甲酯(DMM,CH 3 OC(O)CH 2 C(O)OCH 3)气相反应的速率系数,给出的值为(3.8±0.4) ×10 –12,cm 3分子–1 s –1)。还研究了DMM的光氧化机理。通过红外光谱鉴定了主要产物,并进行了计算计算以支持实验数据。结果表明,光氧化主要是通过从甲基中提取一个H原子而发生的。CH 3 OC(O)CH 2 C(O)OCH 2 O •随后形成的自由基根据三种竞争途径进行反应:与分子氧反应生成CH 3 OC(O)CH 2 C(O)OC(O)H,异构化-单分子分解最终导致CH 3 OC(O)C( O)H,CO 2和HC(O)OH,以及α-酯重排形成丙二酸单甲酯和一氧化碳。还确定了产物收率随氧气压力的变化。
更新日期:2017-11-08
中文翻译:

气相中氯原子引发的丙二酸二甲酯的光氧化反应:动力学和机理
使用相关方法在298 K下确定氯原子与丙二酸二甲酯(DMM,CH 3 OC(O)CH 2 C(O)OCH 3)气相反应的速率系数,给出的值为(3.8±0.4) ×10 –12,cm 3分子–1 s –1)。还研究了DMM的光氧化机理。通过红外光谱鉴定了主要产物,并进行了计算计算以支持实验数据。结果表明,光氧化主要是通过从甲基中提取一个H原子而发生的。CH 3 OC(O)CH 2 C(O)OCH 2 O •随后形成的自由基根据三种竞争途径进行反应:与分子氧反应生成CH 3 OC(O)CH 2 C(O)OC(O)H,异构化-单分子分解最终导致CH 3 OC(O)C( O)H,CO 2和HC(O)OH,以及α-酯重排形成丙二酸单甲酯和一氧化碳。还确定了产物收率随氧气压力的变化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号