当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Products from the Oxidation of n-Butane from 298 to 735 K Using Either Cl Atom or Thermal Initiation: Formation of Acetone and Acetic Acid—Possible Roaming Reactions?
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.jpca.7b06608 E. W. Kaiser 1 , T. J. Wallington 2
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.jpca.7b06608 E. W. Kaiser 1 , T. J. Wallington 2
Affiliation
|
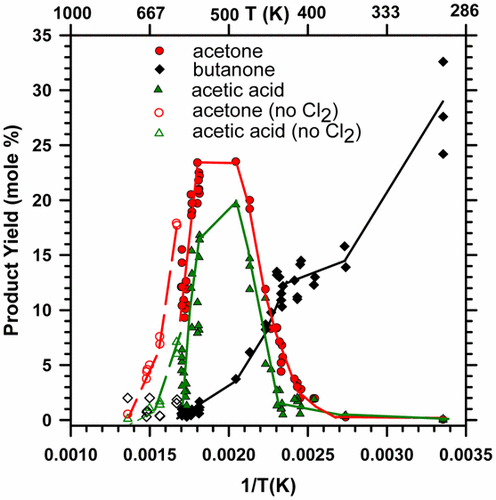
The oxidation of 2-butyl radicals (and to a lesser extent 1-butyl radicals) has been studied over the temperature range of 298–735 K. The reaction of Cl atoms (formed by 360 nm irradiation of Cl2) with n-butane generated the 2-butyl radicals in mixtures of n-C4H10, O2, and Cl2 at temperatures below 600 K. Above 600 K, 2-butyl radicals were produced by thermal combustion reactions in the absence of chlorine. The yields of the products were measured by gas chromatography using a flame ionization detector. Major products quantified include acetone, acetic acid, acetaldehyde, butanone, 2-butanol, butanal, 1- and 2- chlorobutane, 1-butene, trans-2-butene, and cis-2-butene. At 298 K, the major oxygenated products are those expected from bimolecular reactions of 2-butylperoxy radicals (butanone, 2-butanol, and acetaldehyde). As the temperature rises to 390 K, the butanone decreases while acetaldehyde increases because of the increased rate of 2-butoxy radical decomposition. Acetone and acetic acid first appear in significant yield near 400 K, and these species rise slowly at first and then sharply, peaking near 525 K at yields of ∼25 and ∼20 mol %, respectively. In the same temperature range (400–525 K), butanone, acetaldehyde, and 2-butanol decrease rapidly. This suggests that acetone and acetic acid may be formed by previously unknown reaction channels of the 2-butylperoxy radical, which are in competition with those that lead to butanone, acetaldehyde, and 2-butanol. Above 570 K, the yields of acetone and acetic acid fall rapidly as the yields of the butenes rise. Experiments varying the Cl atom density, which in turn controls the entire radical pool density, were performed in the temperature range of 410–440 K. Decreasing the Cl atom density increased the yields of acetone and acetic acid while the yields of butanone, acetaldehyde, and 2-butanol decreased. This is consistent with the formation of acetone and acetic acid by unimolecular decomposition channels of the 2-butylperoxy radical, which are in competition with the bimolecular channels that form butanone, acetaldehyde, and 2-butanol. Such unimolecular decomposition channels would be unlikely to proceed through conventional transition states because those states would be very constrained. Therefore, the possibility that these decomposition channels proceed via roaming should be considered. In addition, we investigated and were unable to fit our data trends by a simplified ketohydroperoxide mechanism.
中文翻译:

使用Cl原子或热引发将正丁烷从298 K氧化为735 K的产物:丙酮和乙酸的形成-可能发生漫游反应吗?
在298–735 K的温度范围内,研究了2-丁基自由基(以及程度较小的1-丁基自由基)的氧化。Cl原子(由Cl 2的360 nm辐照形成)与正丁烷的反应在低于600 K的温度下,会在n -C 4 H 10,O 2和Cl 2的混合物中生成2-丁基自由基。在600 K以上时,在不存在氯的情况下通过热燃烧反应会生成2-丁基自由基。使用火焰离子化检测器通过气相色谱法测量产物的产率。量化的主要产品包括丙酮,乙酸,乙醛,丁酮,2-丁醇,丁醛,1-和2-氯丁烷,1-丁烯,反式-2-丁烯和顺式-2-丁烯。在298 K时,主要的氧化产物是2-丁基过氧自由基(丁酮,2-丁醇和乙醛)的双分子反应所预期的那些。随着温度升高至390 K,由于2-丁氧基自由基分解速率的增加,丁酮减少而乙醛增加。丙酮和乙酸在400 K附近首先以明显的收率出现,并且这些物种先缓慢上升,然后急剧上升,在525 K附近达到峰值,分别收率约为25和20 mol%。在相同温度范围(400–525 K)中,丁酮,乙醛和2-丁醇迅速降低。这表明丙酮和乙酸可以由2-丁基过氧自由基的先前未知的反应通道形成,该反应通道与导致丁酮,乙醛和2-丁醇的那些竞争。570 K以上,随着丁烯收率的提高,丙酮和乙酸的收率迅速下降。在410–440 K的温度范围内进行了改变Cl原子密度的实验,从而控制了整个自由基池的密度。降低Cl原子密度可提高丙酮和乙酸的产率,而丁酮,乙醛,和2-丁醇减少。这与2-丁基过氧自由基的单分子分解通道与形成丁酮,乙醛和2-丁醇的双分子通道竞争形成丙酮和乙酸是一致的。这样的非分子分解通道不可能通过常规的过渡态进行,因为那些态将受到很大的约束。所以,应考虑这些分解通道通过漫游进行的可能性。此外,我们通过简化的酮氢过氧化物机理进行了调查,但无法适应我们的数据趋势。
更新日期:2017-11-08
中文翻译:

使用Cl原子或热引发将正丁烷从298 K氧化为735 K的产物:丙酮和乙酸的形成-可能发生漫游反应吗?
在298–735 K的温度范围内,研究了2-丁基自由基(以及程度较小的1-丁基自由基)的氧化。Cl原子(由Cl 2的360 nm辐照形成)与正丁烷的反应在低于600 K的温度下,会在n -C 4 H 10,O 2和Cl 2的混合物中生成2-丁基自由基。在600 K以上时,在不存在氯的情况下通过热燃烧反应会生成2-丁基自由基。使用火焰离子化检测器通过气相色谱法测量产物的产率。量化的主要产品包括丙酮,乙酸,乙醛,丁酮,2-丁醇,丁醛,1-和2-氯丁烷,1-丁烯,反式-2-丁烯和顺式-2-丁烯。在298 K时,主要的氧化产物是2-丁基过氧自由基(丁酮,2-丁醇和乙醛)的双分子反应所预期的那些。随着温度升高至390 K,由于2-丁氧基自由基分解速率的增加,丁酮减少而乙醛增加。丙酮和乙酸在400 K附近首先以明显的收率出现,并且这些物种先缓慢上升,然后急剧上升,在525 K附近达到峰值,分别收率约为25和20 mol%。在相同温度范围(400–525 K)中,丁酮,乙醛和2-丁醇迅速降低。这表明丙酮和乙酸可以由2-丁基过氧自由基的先前未知的反应通道形成,该反应通道与导致丁酮,乙醛和2-丁醇的那些竞争。570 K以上,随着丁烯收率的提高,丙酮和乙酸的收率迅速下降。在410–440 K的温度范围内进行了改变Cl原子密度的实验,从而控制了整个自由基池的密度。降低Cl原子密度可提高丙酮和乙酸的产率,而丁酮,乙醛,和2-丁醇减少。这与2-丁基过氧自由基的单分子分解通道与形成丁酮,乙醛和2-丁醇的双分子通道竞争形成丙酮和乙酸是一致的。这样的非分子分解通道不可能通过常规的过渡态进行,因为那些态将受到很大的约束。所以,应考虑这些分解通道通过漫游进行的可能性。此外,我们通过简化的酮氢过氧化物机理进行了调查,但无法适应我们的数据趋势。



























 京公网安备 11010802027423号
京公网安备 11010802027423号