当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Metal-Ion- and Hydrogen-Bond-Mediated Interstellar Prebiotic Chemistry: The First Step in the Formose Reaction
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.jpca.7b08002 Sorakayala Thripati 1 , Raghunath O. Ramabhadran 1
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.jpca.7b08002 Sorakayala Thripati 1 , Raghunath O. Ramabhadran 1
Affiliation
|
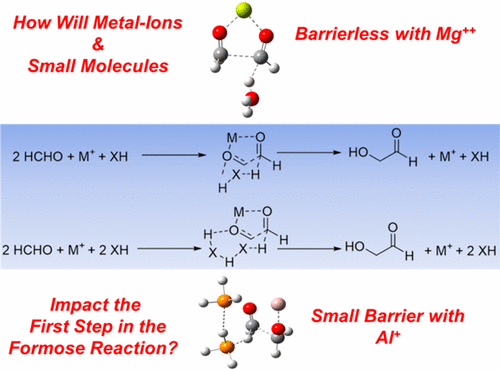
The formose reaction, which offers a feasible chemical pathway for the prebiotic synthesis of sugars, is a well-studied reaction for over two hundred and 50 years. Yet huge knowledge gaps exist even in the very first step of the formose reaction. In this work, we provide a new and otherwise unintuitive reaction pathway for the gas-phase conversion of formaldehyde to glycolaldehyde (the first step in the formose reaction) occurring in the interstellar medium (ISM). Employing electronic structure calculations (CCSD(T) and DFT methods), we exhaustively probe the role of various metal ions and small molecules detected in the ISM to propose a new mechanism wherein metal–oxygen interactions and hydrogen bonds cooperatively facilitate an otherwise implausible chemical reaction. The reactions involving Mg2+ are throughout found to be barrierless, and those featuring Al+ ions are noted to only have a small barrier. The proton affinities of the small molecules, metal–oxygen interactions, and the extent of C–C-bond formation are found to be the significant factors that influence the barrier heights. The mechanism is also shown to be consistent with well-known experimental details in the terrestrial formose reaction (which could, however, proceed through a different mechanism). Future experimental and theoretical scope arising out of this paper are subsequently discussed.
中文翻译:

金属离子和氢键介导的星际益生元间化学反应:甲醛反应的第一步
甲醛反应为益生元糖的合成提供了可行的化学途径,是经过250多年深入研究的反应。但是,即使在formose反应的第一步中,仍然存在巨大的知识鸿沟。在这项工作中,我们为星际介质(ISM)中发生的甲醛到气相甲醛的气相转化(甲糖反应的第一步)提供了一条新颖而又不直观的反应途径。利用电子结构计算(CCSD(T)和DFT方法),我们详尽地探讨了在ISM中检测到的各种金属离子和小分子的作用,从而提出了一种新的机制,其中金属-氧相互作用和氢键共同促进了否则难以置信的化学反应。涉及Mg 2+的反应整个过程都被发现是无障碍的,而那些以Al +离子为特征的分子则只有很小的势垒。发现小分子的质子亲和力,金属-氧相互作用以及C-C键形成的程度是影响势垒高度的重要因素。还显示了该机理与陆基甲糖反应中众所周知的实验细节相一致(但是,可以通过不同的机理进行)。随后对本文提出的未来实验和理论范围进行了讨论。
更新日期:2017-11-08
中文翻译:

金属离子和氢键介导的星际益生元间化学反应:甲醛反应的第一步
甲醛反应为益生元糖的合成提供了可行的化学途径,是经过250多年深入研究的反应。但是,即使在formose反应的第一步中,仍然存在巨大的知识鸿沟。在这项工作中,我们为星际介质(ISM)中发生的甲醛到气相甲醛的气相转化(甲糖反应的第一步)提供了一条新颖而又不直观的反应途径。利用电子结构计算(CCSD(T)和DFT方法),我们详尽地探讨了在ISM中检测到的各种金属离子和小分子的作用,从而提出了一种新的机制,其中金属-氧相互作用和氢键共同促进了否则难以置信的化学反应。涉及Mg 2+的反应整个过程都被发现是无障碍的,而那些以Al +离子为特征的分子则只有很小的势垒。发现小分子的质子亲和力,金属-氧相互作用以及C-C键形成的程度是影响势垒高度的重要因素。还显示了该机理与陆基甲糖反应中众所周知的实验细节相一致(但是,可以通过不同的机理进行)。随后对本文提出的未来实验和理论范围进行了讨论。



























 京公网安备 11010802027423号
京公网安备 11010802027423号