当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Shock Tube and Laser Absorption Study of CH2O Oxidation via Simultaneous Measurements of OH and CO
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.jpca.7b09362 Shengkai Wang 1 , David F. Davidson 1 , Ronald K. Hanson 1
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.jpca.7b09362 Shengkai Wang 1 , David F. Davidson 1 , Ronald K. Hanson 1
Affiliation
|
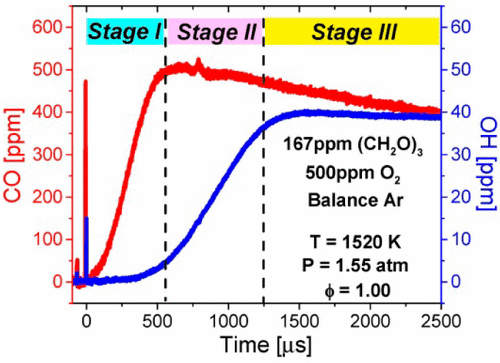
The oxidation of Ar-diluted stoichiometric CH2O–O2 mixtures was studied behind reflected shock waves over temperatures of 1332–1685 K, at pressures of about 1.5 atm and initial CH2O mole fractions of 500, 1500, and 5000 ppm. Quantitative and time-resolved concentration histories of OH and CO (at both v″ = 0 and v″ = 1) were measured by narrow-linewidth laser absorption at 306.7 and 4854 nm, respectively. A time delay was observed between the formation of v″ = 0 and v″ = 1 states of CO, suggesting that CO was kinetically generated primarily in the ground state and then collisionally relaxed toward vibrational equilibrium. The measured CO and OH time-histories were used to evaluate the performance of four detailed reaction mechanisms regarding the oxidation chemistry of CH2O. Further analyses of these time-history data have also led to improved determination for the rate constants of two key reactions, namely H + O2 = O + OH (R1) and OH + CO = CO2 + H (R2), as follows: k1 = 8.04 × 1013 exp(−7370 K/T) cm3 mol–1 s–1, k2 = 1.90 × 1012 exp(−2760 K/T) cm3 mol–1 s–1; both expressions are valid over 1428–1685 K and have 1σ uncertainties of approximately ±10%.
中文翻译:

同时测量OH和CO对CH 2 O氧化的冲击管和激光吸收研究
在1332–1685 K的温度,约1.5 atm的压力和初始CH 2 O摩尔分数分别为500、1500和5000 ppm的情况下,在反射冲击波后,研究了Ar稀释的化学计量CH 2 O–O 2混合物的氧化。通过窄线宽激光吸收在306.7和4854 nm处分别测量OH和CO的定量和时间分辨浓度历史(在v ” = 0和v ” = 1时)。在v ''= 0到v的形成之间观察到时间延迟” = CO的1个状态,表明CO主要是在基态中动力学生成的,然后向振动平衡方向碰撞松弛。使用测得的CO和OH时间历史记录来评估关于CH 2 O氧化化学的四种详细反应机理的性能。对这些时间历史记录数据的进一步分析也导致对两个关键反应速率常数的测定得到了改进,即H + O 2 = O + OH(R1)和OH + CO = CO 2 + H(R2),如下:k 1 = 8.04×10 13 exp(−7370 K / T)cm 3 mol –1 s –1,k 2 = 1.90×10 12exp(−2760 K / T)cm 3摩尔–1 s –1 ; 这两个表达式在1428–1685 K范围内均有效,并且1σ不确定度约为±10%。
更新日期:2017-11-08
中文翻译:

同时测量OH和CO对CH 2 O氧化的冲击管和激光吸收研究
在1332–1685 K的温度,约1.5 atm的压力和初始CH 2 O摩尔分数分别为500、1500和5000 ppm的情况下,在反射冲击波后,研究了Ar稀释的化学计量CH 2 O–O 2混合物的氧化。通过窄线宽激光吸收在306.7和4854 nm处分别测量OH和CO的定量和时间分辨浓度历史(在v ” = 0和v ” = 1时)。在v ''= 0到v的形成之间观察到时间延迟” = CO的1个状态,表明CO主要是在基态中动力学生成的,然后向振动平衡方向碰撞松弛。使用测得的CO和OH时间历史记录来评估关于CH 2 O氧化化学的四种详细反应机理的性能。对这些时间历史记录数据的进一步分析也导致对两个关键反应速率常数的测定得到了改进,即H + O 2 = O + OH(R1)和OH + CO = CO 2 + H(R2),如下:k 1 = 8.04×10 13 exp(−7370 K / T)cm 3 mol –1 s –1,k 2 = 1.90×10 12exp(−2760 K / T)cm 3摩尔–1 s –1 ; 这两个表达式在1428–1685 K范围内均有效,并且1σ不确定度约为±10%。



























 京公网安备 11010802027423号
京公网安备 11010802027423号