当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Organocatalytic Asymmetric Allylic Alkylation of Morita–Baylis–Hillman Carbonates with Diethyl 2-Aminomalonate Assisted by In Situ Protection
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.joc.7b02064 Yu Zheng 1 , Jing Wang 1 , Peng-Ju Xia 1 , Qing-lan Zhao 1 , Jun-An Xiao 2 , Hao-Yue Xiang 1 , Xiao-Qing Chen 1 , Hua Yang 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.joc.7b02064 Yu Zheng 1 , Jing Wang 1 , Peng-Ju Xia 1 , Qing-lan Zhao 1 , Jun-An Xiao 2 , Hao-Yue Xiang 1 , Xiao-Qing Chen 1 , Hua Yang 1
Affiliation
|
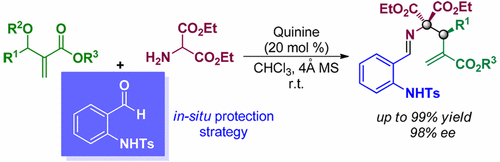
With the aid of in situ protection by N-(2-formylphenyl)-4-methyl-benzenesulfonamide, enantioselective allylic alkylation of Morita–Baylis–Hillman carbonates with diethyl 2-aminomalonate was successfully realized. The corresponding adducts can be obtained in up to 99% yield with up to 98% ee as well as excellent regioselectivity. Besides, the adducts with opposite configurations were readily prepared by utilizing easily available and inexpensive quinine or quinidine as organocatalyst. Facile deprotection of the resulting adduct provides straightforward access to enantiopure α-methylene-γ-lactam.
中文翻译:

Morita–Baylis–Hillman碳酸盐的有机催化不对称烯丙基烷基化与2-氨基壬二酸二乙酯的原位保护
借助N-(2-甲酰基苯基)-4-甲基-苯磺酰胺的原位保护,成功实现了森田-贝利斯-希尔曼碳酸盐与2-氨基丙二酸二乙酯的对映选择性烯丙基烷基化反应。可以以高达99%的收率和高达98%的ee以及相应的优异的区域选择性获得相应的加合物。此外,具有相反构型的加合物可以通过利用容易获得且便宜的奎宁或奎尼丁作为有机催化剂来制备。容易地脱保护所得的加合物,可直接获得对映纯的α-亚甲基-γ-内酰胺。
更新日期:2017-11-08
中文翻译:

Morita–Baylis–Hillman碳酸盐的有机催化不对称烯丙基烷基化与2-氨基壬二酸二乙酯的原位保护
借助N-(2-甲酰基苯基)-4-甲基-苯磺酰胺的原位保护,成功实现了森田-贝利斯-希尔曼碳酸盐与2-氨基丙二酸二乙酯的对映选择性烯丙基烷基化反应。可以以高达99%的收率和高达98%的ee以及相应的优异的区域选择性获得相应的加合物。此外,具有相反构型的加合物可以通过利用容易获得且便宜的奎宁或奎尼丁作为有机催化剂来制备。容易地脱保护所得的加合物,可直接获得对映纯的α-亚甲基-γ-内酰胺。


























 京公网安备 11010802027423号
京公网安备 11010802027423号