当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Nucleophilic Addition of Ketones To Acetylenes and Allenes: A Quantum-Chemical Insight
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.joc.7b02263 Nadezhda M. Vitkovskaya 1 , Vladimir B. Kobychev 1 , Alexander S. Bobkov 1 , Vladimir B. Orel 1 , Elena Yu. Schmidt 2 , Boris A. Trofimov 2
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.joc.7b02263 Nadezhda M. Vitkovskaya 1 , Vladimir B. Kobychev 1 , Alexander S. Bobkov 1 , Vladimir B. Orel 1 , Elena Yu. Schmidt 2 , Boris A. Trofimov 2
Affiliation
|
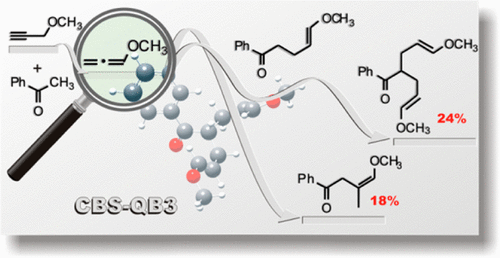
A CBS-Q//B3 based study has been carried out to elucidate the mechanism of the KOH/DMSO superbase catalyzed ketones nucleophilic addition to alkyl propargyl and alkyl allenyl ethers yielding, along with (Z)-monoadducts, up to 26% of unexpected (E)-diadducts. The impact of different substrates (alkynes versus allenes) on the reaction mechanism has been discussed in detail. Along with the model reaction of acetone addition to propyne and allene, the addition of acetone and acetophenone to methyl propargyl and methyl allenyl ethers is considered. The limiting reaction stage of the starting ketone carbanion addition to propargyl and allenyl systems occurs with activation energies typical for vinylation of ketones. In contrast, the addition of intermediate α-carbanions to the terminal position of methyl allenyl ether is associated with unusually low activation barriers. The results obtained explain the composition of the reaction products and indicate the participation of mainly the allene form in the reaction.
中文翻译:

酮对乙炔和丙二烯的亲核加成:量子化学的见解。
已经进行了一项基于CBS-Q // B3的研究,以阐明KOH / DMSO超碱催化的酮与烷基炔丙基和烷基烯基醚的亲核加成反应产生的机理,以及(Z)-单加合物的产生率高达26%。 (E)-高架桥。已经详细讨论了不同底物(炔烃与丙二烯)对反应机理的影响。伴随着丙酮与丙炔和丙二烯加成反应的模型反应,还考虑了向甲基炔丙基和甲基烯丙基醚中加成丙酮和苯乙酮。起始酮碳负离子加到炔丙基和烯丙基系统中的极限反应阶段发生在酮的乙烯基化过程中典型的活化能上。相比之下,在甲基烯丙基醚的末端位置添加中间体α-碳负离子与异常低的活化势垒相关。所获得的结果解释了反应产物的组成,并表明反应中主要是烯丙基形式的参与。
更新日期:2017-11-08
中文翻译:

酮对乙炔和丙二烯的亲核加成:量子化学的见解。
已经进行了一项基于CBS-Q // B3的研究,以阐明KOH / DMSO超碱催化的酮与烷基炔丙基和烷基烯基醚的亲核加成反应产生的机理,以及(Z)-单加合物的产生率高达26%。 (E)-高架桥。已经详细讨论了不同底物(炔烃与丙二烯)对反应机理的影响。伴随着丙酮与丙炔和丙二烯加成反应的模型反应,还考虑了向甲基炔丙基和甲基烯丙基醚中加成丙酮和苯乙酮。起始酮碳负离子加到炔丙基和烯丙基系统中的极限反应阶段发生在酮的乙烯基化过程中典型的活化能上。相比之下,在甲基烯丙基醚的末端位置添加中间体α-碳负离子与异常低的活化势垒相关。所获得的结果解释了反应产物的组成,并表明反应中主要是烯丙基形式的参与。


























 京公网安备 11010802027423号
京公网安备 11010802027423号