当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of Allahabadolactone A
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.joc.7b02368 Kongchen Wang 1 , Roderick W. Bates 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.joc.7b02368 Kongchen Wang 1 , Roderick W. Bates 1
Affiliation
|
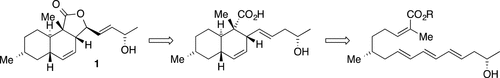
A synthesis of allahabadolactone A is described employing diastereoselective Diels–Alder and selenocyclization reactions, starting from (R)-citronellal and propylene oxide. The Diels–Alder substrate is built up in an efficient manner by rhodium-catalyzed alkyne hydroboration and palladium-catalyzed coupling reactions of E-1,2-dichloroethene. It is observed that the Diels–Alder reaction only displays high diastereoselectivity when the diene bears an additional alkene substituent but not an alkyne substituent.
中文翻译:

阿拉哈巴内酯A的合成
描述了采用非对映选择性Diels-Alder和硒环化反应(从(R)-香茅醛和环氧丙烷开始)合成阿拉巴巴内酯A的方法。Diels–Alder底物通过铑催化的炔烃硼氢化反应和钯催化的E -1,2-二氯乙烯偶联反应而高效地构建。可以观察到,当二烯带有一个额外的烯烃取代基而不是一个炔烃取代基时,狄尔斯-阿尔德反应仅表现出高的非对映选择性。
更新日期:2017-11-08
中文翻译:

阿拉哈巴内酯A的合成
描述了采用非对映选择性Diels-Alder和硒环化反应(从(R)-香茅醛和环氧丙烷开始)合成阿拉巴巴内酯A的方法。Diels–Alder底物通过铑催化的炔烃硼氢化反应和钯催化的E -1,2-二氯乙烯偶联反应而高效地构建。可以观察到,当二烯带有一个额外的烯烃取代基而不是一个炔烃取代基时,狄尔斯-阿尔德反应仅表现出高的非对映选择性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号