当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Application of the EF and GH Fragments to the Synthesis of Idraparinux
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.joc.7b02497 Grzegorz Łopatkiewicz 1 , Szymon Buda 1 , Jacek Mlynarski 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.joc.7b02497 Grzegorz Łopatkiewicz 1 , Szymon Buda 1 , Jacek Mlynarski 1
Affiliation
|
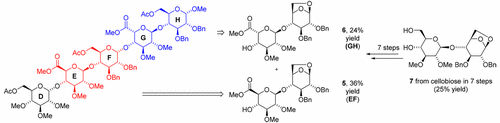
A novel total synthesis of fully protected idraparinux has been achieved. A short and efficient protocol for the synthesis of the EF fragment of idraparinux and its C5′-epi analogue (GH unit) has been developed. The same cellobiose unit was transformed in 14 steps into the fully protected EF and GH disaccharide fragments. The key step of this approach is an epimerization of C5 by an elimination–addition sequence leading to l-ido disaccharide (GH unit) with a total yield of 24% (36% for the EF fragment). 1,6-Anhydro ring opening gave suitable substrates for efficient synthesis of fully protected idraparinux. The fully protected antithrombotic pentasaccharide idraparinux was synthesized in 23 steps for the longest linear route, with a 1.7% overall yield from d-cellobiose and d-glucose.
中文翻译:

EF和GH片段在Idraparinux合成中的应用
已经实现了完全保护的伊德拉帕林的新颖的全合成。已经开发了用于合成伊德拉帕林的EF片段及其C5'- epi类似物(GH单位)的短而有效的方案。相同的纤维二糖单元经过14步转化为完全保护的EF和GH二糖片段。该方法的关键步骤是通过消除加成序列使C5发生差向异构化,从而产生l - ido二糖(GH单位),总产率为24%(EF为36%)分段)。1,6-脱水环开环提供了合适的底物,可用于高效合成完全受保护的伊德拉帕林。完全保护的五糖的抗血栓艾屈肝素在23步为最长线性路线合成,与来自1.7%的总收率d -cellobiose和d -葡萄糖。
更新日期:2017-11-08
中文翻译:

EF和GH片段在Idraparinux合成中的应用
已经实现了完全保护的伊德拉帕林的新颖的全合成。已经开发了用于合成伊德拉帕林的EF片段及其C5'- epi类似物(GH单位)的短而有效的方案。相同的纤维二糖单元经过14步转化为完全保护的EF和GH二糖片段。该方法的关键步骤是通过消除加成序列使C5发生差向异构化,从而产生l - ido二糖(GH单位),总产率为24%(EF为36%)分段)。1,6-脱水环开环提供了合适的底物,可用于高效合成完全受保护的伊德拉帕林。完全保护的五糖的抗血栓艾屈肝素在23步为最长线性路线合成,与来自1.7%的总收率d -cellobiose和d -葡萄糖。



























 京公网安备 11010802027423号
京公网安备 11010802027423号