当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Dual nickel and Lewis acid catalysis for cross-electrophile coupling: the allylation of aryl halides with allylic alcohols†
Chemical Science ( IF 8.4 ) Pub Date : 2017-11-06 00:00:00 , DOI: 10.1039/c7sc03140h Xue-Gong Jia 1 , Peng Guo 1 , Jicheng Duan 1 , Xing-Zhong Shu 1
Chemical Science ( IF 8.4 ) Pub Date : 2017-11-06 00:00:00 , DOI: 10.1039/c7sc03140h Xue-Gong Jia 1 , Peng Guo 1 , Jicheng Duan 1 , Xing-Zhong Shu 1
Affiliation
|
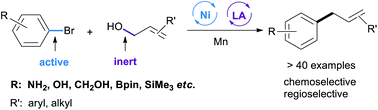
Controlling the selectivity in cross-electrophile coupling reactions is a significant challenge, particularly when one electrophile is much more reactive. We report a general and practical strategy to address this problem in the reaction between reactive and unreactive electrophiles by a combination of nickel and Lewis acid catalysis. This strategy is used for the coupling of aryl halides with allylic alcohols to form linear allylarenes selectively. The reaction tolerates a wide range of functional groups (e.g. silanes, boronates, anilines, esters, alcohols, and various heterocycles) and works with various allylic alcohols. Complementary to most current routes for the C3 allylation of an unprotected indole, this method provides access to C2 and C4–C7 allylated indoles. Preliminary mechanistic experiments reveal that the reaction might start with an aryl nickel intermediate, which then reacts with Lewis acid activated allylic alcohols in the presence of Mn.
中文翻译:

用于交叉亲电偶联的双镍和路易斯酸催化:芳基卤化物与烯丙醇的烯丙基化†
控制交叉亲电偶联反应的选择性是一项重大挑战,特别是当一种亲电试剂反应性更强时。我们报告了一种通用且实用的策略,通过镍和路易斯酸催化的组合来解决反应性和非反应性亲电试剂之间的反应中的这个问题。该策略用于将芳基卤化物与烯丙醇偶联以选择性地形成线性烯丙芳烃。该反应耐受广泛的官能团(例如硅烷、硼酸盐、苯胺、酯、醇和各种杂环)并与各种烯丙醇一起使用。作为对未受保护的吲哚的 C3 烯丙基化的大多数当前路线的补充,该方法提供了对 C2 和 C4-C7 烯丙基化吲哚的访问。初步的机理实验表明,该反应可能从芳基镍中间体开始,然后在 Mn 存在下与路易斯酸活化的烯丙醇反应。
更新日期:2017-11-06
中文翻译:

用于交叉亲电偶联的双镍和路易斯酸催化:芳基卤化物与烯丙醇的烯丙基化†
控制交叉亲电偶联反应的选择性是一项重大挑战,特别是当一种亲电试剂反应性更强时。我们报告了一种通用且实用的策略,通过镍和路易斯酸催化的组合来解决反应性和非反应性亲电试剂之间的反应中的这个问题。该策略用于将芳基卤化物与烯丙醇偶联以选择性地形成线性烯丙芳烃。该反应耐受广泛的官能团(例如硅烷、硼酸盐、苯胺、酯、醇和各种杂环)并与各种烯丙醇一起使用。作为对未受保护的吲哚的 C3 烯丙基化的大多数当前路线的补充,该方法提供了对 C2 和 C4-C7 烯丙基化吲哚的访问。初步的机理实验表明,该反应可能从芳基镍中间体开始,然后在 Mn 存在下与路易斯酸活化的烯丙醇反应。


























 京公网安备 11010802027423号
京公网安备 11010802027423号