Bioorganic & Medicinal Chemistry ( IF 3.5 ) Pub Date : 2017-11-04 , DOI: 10.1016/j.bmc.2017.10.046 Ding Li , Tuong Thi Mai Luong , Wen-Jia Dan , Yanliang Ren , Hoang Xuan Nien , An-Ling Zhang , Jin-Ming Gao
|
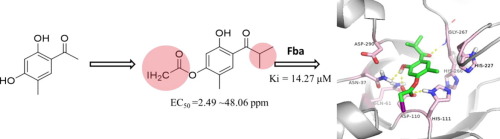
Several recently identified antifungal compounds share the backbone structure of acetophenones. The aim of the present study was to develop new isobutyrophenone analogs as new antifungal agents. A series of new 2,4-dihydroxy-5-methyl isobutyrophenone derivatives were prepared and characterized by 1H, 13C NMR and MS spectroscopic data. These products were evaluated for in vitro antifungal activities against seven plant fungal pathogens by the mycelial growth inhibitory rate assay. Compounds 3, 4a, 5a, 5b, 5e, 5f and 5g showed a broad-spectrum high antifungal activity. On the other hand, for the first time, these compounds were also assayed as potential inhibitors against Class II fructose-1,6-bisphosphate aldolase (Fba) from the rice blast fungus, Magnaporthe grisea. Compounds 5e and 5g were found to exhibit the inhibition constants (Ki) for 15.12 and 14.27 μM, respectively, as the strongest competitive inhibitors against Fba activity. The possible binding-modes of compounds 5e and 5g were further analyzed by molecular docking algorithms. The results strongly suggested that compound 5g could be a promising lead for the discovery of new fungicides via targeting Class II Fba.
中文翻译:

天然产物作为新的杀菌剂的来源(四):异丁苯酮类似物作为II类果糖-1,6-二磷酸醛缩酶的潜在抑制剂的合成和生物学评估
最近发现的几种抗真菌化合物共有苯乙酮的骨架结构。本研究的目的是开发新的异丁苯酮类似物作为新的抗真菌剂。制备了一系列新的2,4-二羟基-5-甲基异丁苯酮衍生物,并通过1 H,13 C NMR和MS光谱数据表征。通过菌丝体生长抑制率测定法评估了这些产品对七种植物真菌病原体的体外抗真菌活性。化合物3,图4a,图5a,图5b,图5e,图5f以及5克表现出广谱的高抗真菌活性。另一方面,这些化合物也首次被鉴定为针对稻瘟病菌Magnaporthe grisea的II类果糖-1,6-双磷酸醛缩酶(Fba)的潜在抑制剂。发现化合物5e和5g作为针对Fba活性的最强竞争性抑制剂,分别显示出15.12和14.27μM的抑制常数(Ki)。通过分子对接算法进一步分析了化合物5e和5g可能的结合方式。结果强烈表明,化合物5g可以通过靶向II类Fba来发现新的杀菌剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号