当前位置:
X-MOL 学术
›
Faraday Discuss.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A QM/MM study of the initial excited state dynamics of green-absorbing proteorhodopsin
Faraday Discussions ( IF 3.4 ) Pub Date : 2017-11-03 , DOI: 10.1039/c7fd00198c Veniamin A. Borin 1, 2, 3, 4, 5 , Christian Wiebeler 1, 2, 3, 4, 5 , Igor Schapiro 1, 2, 3, 4, 5
Faraday Discussions ( IF 3.4 ) Pub Date : 2017-11-03 , DOI: 10.1039/c7fd00198c Veniamin A. Borin 1, 2, 3, 4, 5 , Christian Wiebeler 1, 2, 3, 4, 5 , Igor Schapiro 1, 2, 3, 4, 5
Affiliation
|
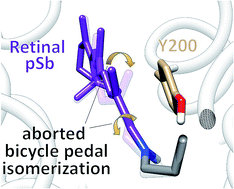
The primary photochemical reaction of the green-absorbing proteorhodopsin is studied by means of a hybrid quantum mechanics/molecular mechanics (QM/MM) approach. The simulations are based on a homology model derived from the blue-absorbing proteorhodopsin crystal structure. The geometry of retinal and the surrounding sidechains in the protein binding pocket were optimized using the QM/MM method. Starting from this geometry the isomerization was studied with a relaxed scan along the C13![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) C14 dihedral. It revealed an “aborted bicycle pedal” mechanism of isomerization that was originally proposed by Warshel for bovine rhodopsin and bacteriorhodopsin. However, the isomerization involved the concerted rotation about C13
C14 dihedral. It revealed an “aborted bicycle pedal” mechanism of isomerization that was originally proposed by Warshel for bovine rhodopsin and bacteriorhodopsin. However, the isomerization involved the concerted rotation about C13![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) C14 and C15
C14 and C15![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) N, with the latter being highly twisted but not isomerized. Further, the simulation showed an increased steric interaction between the hydrogen at the C14 of the isomerizing bond and the hydroxyl group at the neighbouring tyrosine 200. In addition, we have simulated a nonadiabatic trajectory which showed the timing of the isomerization. In the first 20 fs upon excitation the order of the conjugated double and single bonds is inverted, consecutively the C13
N, with the latter being highly twisted but not isomerized. Further, the simulation showed an increased steric interaction between the hydrogen at the C14 of the isomerizing bond and the hydroxyl group at the neighbouring tyrosine 200. In addition, we have simulated a nonadiabatic trajectory which showed the timing of the isomerization. In the first 20 fs upon excitation the order of the conjugated double and single bonds is inverted, consecutively the C13![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) C14 rotation is activated for 200 fs until the S1–S0 transition is detected. However, the isomerization is reverted due to the specific interaction with the tyrosine as observed along the relaxed scan calculation. Our simulations indicate that the retinal – tyrosine 200 interaction plays an important role in the outcome of the photoisomerization.
C14 rotation is activated for 200 fs until the S1–S0 transition is detected. However, the isomerization is reverted due to the specific interaction with the tyrosine as observed along the relaxed scan calculation. Our simulations indicate that the retinal – tyrosine 200 interaction plays an important role in the outcome of the photoisomerization.
中文翻译:

QM / MM研究绿色吸收蛋白视紫红质的初始激发态动力学
通过混合量子力学/分子力学(QM / MM)方法研究了绿色吸收蛋白视紫红质的主要光化学反应。该模拟基于从吸收蓝色的蛋白视紫红质晶体结构衍生的同源性模型。使用QM / MM方法优化了蛋白质结合袋中的视网膜和周围侧链的几何结构。从这种几何形状开始,通过沿C 13![[双键,长度为m-破折号]](http://www.rsc.org/images/entities/char_e001.gif) C 14二面体的松弛扫描研究了异构化。它揭示了Warshel最初提出的用于牛视紫红质和细菌视紫红质的“流离失所的自行车踏板”异构化机制。但是,异构化涉及绕C 13
C 14二面体的松弛扫描研究了异构化。它揭示了Warshel最初提出的用于牛视紫红质和细菌视紫红质的“流离失所的自行车踏板”异构化机制。但是,异构化涉及绕C 13![[双键,长度为m-破折号]](http://www.rsc.org/images/entities/char_e001.gif) C 14和C的协同旋转。15
C 14和C的协同旋转。15![[双键,长度为m-破折号]](http://www.rsc.org/images/entities/char_e001.gif) N,后者高度扭曲但未异构化。此外,模拟显示异构化键的C 14处的氢与相邻酪氨酸200处的羟基之间的空间相互作用增加。此外,我们还模拟了非绝热轨迹,该轨迹显示了异构化的时间。在激发的前20 fs中,共轭双键和单键的顺序反转,随后C 13
N,后者高度扭曲但未异构化。此外,模拟显示异构化键的C 14处的氢与相邻酪氨酸200处的羟基之间的空间相互作用增加。此外,我们还模拟了非绝热轨迹,该轨迹显示了异构化的时间。在激发的前20 fs中,共轭双键和单键的顺序反转,随后C 13![[双键,长度为m-破折号]](http://www.rsc.org/images/entities/char_e001.gif) C 14旋转被激活200 fs,直到S 1 –S 0检测到转变。然而,由于沿着松弛扫描计算观察到的与酪氨酸的特异性相互作用,异构化得以恢复。我们的模拟表明,视网膜–酪氨酸200的相互作用在光异构化的结果中起着重要的作用。
C 14旋转被激活200 fs,直到S 1 –S 0检测到转变。然而,由于沿着松弛扫描计算观察到的与酪氨酸的特异性相互作用,异构化得以恢复。我们的模拟表明,视网膜–酪氨酸200的相互作用在光异构化的结果中起着重要的作用。
更新日期:2018-04-17
![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) C14 dihedral. It revealed an “aborted bicycle pedal” mechanism of isomerization that was originally proposed by Warshel for bovine rhodopsin and bacteriorhodopsin. However, the isomerization involved the concerted rotation about C13
C14 dihedral. It revealed an “aborted bicycle pedal” mechanism of isomerization that was originally proposed by Warshel for bovine rhodopsin and bacteriorhodopsin. However, the isomerization involved the concerted rotation about C13![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) C14 and C15
C14 and C15![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) N, with the latter being highly twisted but not isomerized. Further, the simulation showed an increased steric interaction between the hydrogen at the C14 of the isomerizing bond and the hydroxyl group at the neighbouring tyrosine 200. In addition, we have simulated a nonadiabatic trajectory which showed the timing of the isomerization. In the first 20 fs upon excitation the order of the conjugated double and single bonds is inverted, consecutively the C13
N, with the latter being highly twisted but not isomerized. Further, the simulation showed an increased steric interaction between the hydrogen at the C14 of the isomerizing bond and the hydroxyl group at the neighbouring tyrosine 200. In addition, we have simulated a nonadiabatic trajectory which showed the timing of the isomerization. In the first 20 fs upon excitation the order of the conjugated double and single bonds is inverted, consecutively the C13![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) C14 rotation is activated for 200 fs until the S1–S0 transition is detected. However, the isomerization is reverted due to the specific interaction with the tyrosine as observed along the relaxed scan calculation. Our simulations indicate that the retinal – tyrosine 200 interaction plays an important role in the outcome of the photoisomerization.
C14 rotation is activated for 200 fs until the S1–S0 transition is detected. However, the isomerization is reverted due to the specific interaction with the tyrosine as observed along the relaxed scan calculation. Our simulations indicate that the retinal – tyrosine 200 interaction plays an important role in the outcome of the photoisomerization.
中文翻译:

QM / MM研究绿色吸收蛋白视紫红质的初始激发态动力学
通过混合量子力学/分子力学(QM / MM)方法研究了绿色吸收蛋白视紫红质的主要光化学反应。该模拟基于从吸收蓝色的蛋白视紫红质晶体结构衍生的同源性模型。使用QM / MM方法优化了蛋白质结合袋中的视网膜和周围侧链的几何结构。从这种几何形状开始,通过沿C 13
![[双键,长度为m-破折号]](http://www.rsc.org/images/entities/char_e001.gif) C 14二面体的松弛扫描研究了异构化。它揭示了Warshel最初提出的用于牛视紫红质和细菌视紫红质的“流离失所的自行车踏板”异构化机制。但是,异构化涉及绕C 13
C 14二面体的松弛扫描研究了异构化。它揭示了Warshel最初提出的用于牛视紫红质和细菌视紫红质的“流离失所的自行车踏板”异构化机制。但是,异构化涉及绕C 13![[双键,长度为m-破折号]](http://www.rsc.org/images/entities/char_e001.gif) C 14和C的协同旋转。15
C 14和C的协同旋转。15![[双键,长度为m-破折号]](http://www.rsc.org/images/entities/char_e001.gif) N,后者高度扭曲但未异构化。此外,模拟显示异构化键的C 14处的氢与相邻酪氨酸200处的羟基之间的空间相互作用增加。此外,我们还模拟了非绝热轨迹,该轨迹显示了异构化的时间。在激发的前20 fs中,共轭双键和单键的顺序反转,随后C 13
N,后者高度扭曲但未异构化。此外,模拟显示异构化键的C 14处的氢与相邻酪氨酸200处的羟基之间的空间相互作用增加。此外,我们还模拟了非绝热轨迹,该轨迹显示了异构化的时间。在激发的前20 fs中,共轭双键和单键的顺序反转,随后C 13![[双键,长度为m-破折号]](http://www.rsc.org/images/entities/char_e001.gif) C 14旋转被激活200 fs,直到S 1 –S 0检测到转变。然而,由于沿着松弛扫描计算观察到的与酪氨酸的特异性相互作用,异构化得以恢复。我们的模拟表明,视网膜–酪氨酸200的相互作用在光异构化的结果中起着重要的作用。
C 14旋转被激活200 fs,直到S 1 –S 0检测到转变。然而,由于沿着松弛扫描计算观察到的与酪氨酸的特异性相互作用,异构化得以恢复。我们的模拟表明,视网膜–酪氨酸200的相互作用在光异构化的结果中起着重要的作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号