当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Development of 5N-Bicalutamide, a High-Affinity Reversible Covalent Antiandrogen
ACS Chemical Biology ( IF 4 ) Pub Date : 2017-11-02 00:00:00 , DOI: 10.1021/acschembio.7b00702 Felipe de Jesus Cortez 1 , Phuong Nguyen 2 , Charles Truillet 3 , Boxue Tian 1 , Kristopher M. Kuchenbecker 2 , Michael J. Evans 3 , Paul Webb 4 , Matthew P. Jacobson 1 , Robert J. Fletterick 2 , Pamela M. England 1
ACS Chemical Biology ( IF 4 ) Pub Date : 2017-11-02 00:00:00 , DOI: 10.1021/acschembio.7b00702 Felipe de Jesus Cortez 1 , Phuong Nguyen 2 , Charles Truillet 3 , Boxue Tian 1 , Kristopher M. Kuchenbecker 2 , Michael J. Evans 3 , Paul Webb 4 , Matthew P. Jacobson 1 , Robert J. Fletterick 2 , Pamela M. England 1
Affiliation
|
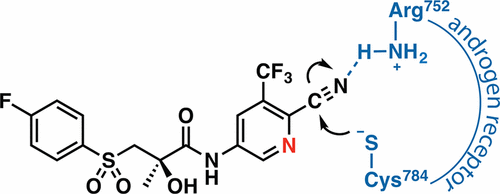
Resistance to clinical antiandrogens has plagued the evolution of effective therapeutics for advanced prostate cancer. As with the first-line therapeutic bicalutamide (Casodex), resistance to newer antiandrogens (enzalutamide, ARN-509) develops quickly in patients, despite the fact that these drugs have ∼10-fold better affinity for the androgen receptor than bicalutamide. Improving affinity alone is often not sufficient to prevent resistance, and alternative strategies are needed to improve antiandrogen efficacy. Covalent and reversible covalent drugs are being used to thwart drug resistance in other contexts, and activated aryl nitriles are among the moieties being exploited for this purpose. We capitalized on the presence of an aryl nitrile in bicalutamide, and the existence of a native cysteine residue (Cys784) in the androgen receptor ligand binding pocket, to develop 5N-bicalutamide, a cysteine-reactive antiandrogen. 5N-bicalutamide exhibits a 150-fold improvement in Ki and 20-fold improvement in IC50 over the parent compound. We attribute the marked improvement in affinity and activity to the formation of a covalent adduct with Cys784, a residue that is not among the more than 160 androgen receptor point mutations associated with prostate cancer. Increasing the residence time of bound antiandrogen via formation of a covalent adduct may forestall the drug resistance seen with current clinical antiandrogens.
中文翻译:

高亲和力可逆共价抗雄激素5N-比卡鲁胺的开发
对临床抗雄激素的耐药性困扰着晚期前列腺癌有效疗法的发展。与一线治疗比卡鲁胺(Casodex)一样,患者对新型抗雄激素药(enzalutamide,ARN-509)的耐药性迅速发展,尽管这些药物对雄激素受体的亲和力比比卡鲁胺好约10倍。仅仅提高亲和力通常不足以预防耐药性,并且需要替代策略来提高抗雄激素功效。在其他情况下,共价和可逆共价药物被用于阻止耐药性,而活化的芳基腈就是为此目的而开发的部分。我们利用比卡鲁胺中存在芳基腈的方法,并在雄激素受体配体结合口袋中存在天然半胱氨酸残基(Cys784),以开发5N-比卡鲁胺,一种半胱氨酸反应性抗雄激素。5N-比卡鲁胺表现出150倍的改善与母体化合物相比,K i和IC 50改善20倍。我们将亲和力和活性的显着改善归因于与Cys784形成共价加合物,该残基不在与前列腺癌相关的超过160个雄激素受体点突变中。通过形成共价加合物来增加结合的抗雄激素的停留时间可能会阻止目前临床抗雄激素所见的耐药性。
更新日期:2017-11-03
中文翻译:

高亲和力可逆共价抗雄激素5N-比卡鲁胺的开发
对临床抗雄激素的耐药性困扰着晚期前列腺癌有效疗法的发展。与一线治疗比卡鲁胺(Casodex)一样,患者对新型抗雄激素药(enzalutamide,ARN-509)的耐药性迅速发展,尽管这些药物对雄激素受体的亲和力比比卡鲁胺好约10倍。仅仅提高亲和力通常不足以预防耐药性,并且需要替代策略来提高抗雄激素功效。在其他情况下,共价和可逆共价药物被用于阻止耐药性,而活化的芳基腈就是为此目的而开发的部分。我们利用比卡鲁胺中存在芳基腈的方法,并在雄激素受体配体结合口袋中存在天然半胱氨酸残基(Cys784),以开发5N-比卡鲁胺,一种半胱氨酸反应性抗雄激素。5N-比卡鲁胺表现出150倍的改善与母体化合物相比,K i和IC 50改善20倍。我们将亲和力和活性的显着改善归因于与Cys784形成共价加合物,该残基不在与前列腺癌相关的超过160个雄激素受体点突变中。通过形成共价加合物来增加结合的抗雄激素的停留时间可能会阻止目前临床抗雄激素所见的耐药性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号