当前位置:
X-MOL 学术
›
Anal. Chim. Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Behavior and kinetic of hydrolysis of amine boranes in acid media employed in chemical vapor generation
Analytica Chimica Acta ( IF 6.2 ) Pub Date : 2018-01-01 , DOI: 10.1016/j.aca.2017.10.034 Lucia D'Ulivo , Roberto Spiniello , Massimo Onor , Beatrice Campanella , Zoltan Mester , Alessandro D'Ulivo
Analytica Chimica Acta ( IF 6.2 ) Pub Date : 2018-01-01 , DOI: 10.1016/j.aca.2017.10.034 Lucia D'Ulivo , Roberto Spiniello , Massimo Onor , Beatrice Campanella , Zoltan Mester , Alessandro D'Ulivo
|
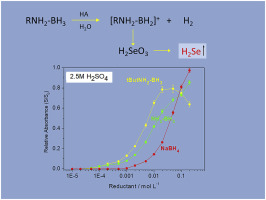
The behavior of NaBH4 (THB) and the amine boranes, NH3BH3 (AB), tertbutylNH2BH3 (TBAB), Me2NHBH3 (DMAB) was investigated in continuous flow chemical vapor generation of H2Se from aqueous SeIV coupled with atomic absorption spectrometry. Unexpected higher efficiency of H2Se generation was obtained with amine boranes compared to THB (TBAB > AB > THB) using millimolar concentration of reductant (0.001-0.1 mol L-1) under strongly acidic conditions (HCl, HClO4, H2SO4, HNO3, 0.5-5 mol L-1 H+). Analytical applicability of the CVG system was tested by the determination of SeIV in natural water samples certified reference materials, using 0.01 mol L-1 TBAB in 0.5 M H2SO4. In order to explain this unexpected higher efficiency of amine boranes with respect of THB, the kinetic of hydrolysis of AB, TBAB and DMAB was investigated in acid media typically employed in chemical vapor generation for trace element determination. The kinetic was investigated by monitoring the rate the hydrogen gas evolved during hydrolysis, using a laboratory made thermostated reaction cell. Kinetics were measured for AB, TBAB and DMAB in 0.1, 0.5, 5 mol L-1 HCl or HClO4 reaction media and in 0.1 mol L-1 cysteine +0.1 mol L-1 HCl or HClO4 buffer, for reaction times from 0 to 30 min. Under strongly acidic conditions, the rates of hydrogen evolution produced by amine boranes hydrolysis appear to be much slower than those predicted by a pseudo-first order reaction and using the rate constants reported in the literature. This suggests that, at elevated acidities (5 mol L-1 HCl or HClO4), the hydrolysis of amine boranes takes place in two steps, generating a first amount of H2 (0.67-1.15 mol) much faster than the remaining about 2 mol. This evidence indicates a different mechanism of hydrolysis to the one accepted in the literature for amine boranes. The relatively high efficiencies of H2Se observed with amine borane reduction of inorganic SeIV at elevated acidities can be addressed to the action of borane intermediates, most probably amine borane cations, formed during amine borane hydrolysis in the same reaction conditions.
中文翻译:

胺硼烷在用于化学蒸汽发生的酸性介质中的水解行为和动力学
NaBH4 (THB) 和胺硼烷、NH3BH3 (AB)、叔丁基 NH2BH3 (TBAB)、Me2NHBH3 (DMAB) 在连续流动化学气相生成 H2Se 的 SeIV 水溶液中结合原子吸收光谱进行了研究。与 THB 相比,使用毫摩尔浓度的还原剂 (0.001-0.1 mol L-1) 在强酸性条件下(HCl、HClO4、H2SO4、HNO3、0.5- 5 mol L-1 H+)。CVG 系统的分析适用性是通过在 0.5 M H2SO4 中使用 0.01 mol L-1 TBAB 测定天然水样认证参考材料中的 SeIV 来测试的。为了解释胺硼烷在 THB 方面出乎意料的更高效率,AB 的水解动力学,TBAB 和 DMAB 在酸性介质中进行了研究,该介质通常用于化学蒸汽发生以测定痕量元素。使用实验室制造的恒温反应池,通过监测水解过程中产生的氢气速率来研究动力学。在 0.1、0.5、5 mol L-1 HCl 或 HClO4 反应介质和 0.1 mol L-1 半胱氨酸 +0.1 mol L-1 HCl 或 HClO4 缓冲液中测量 AB、TBAB 和 DMAB 的动力学,反应时间为 0 到 30分钟 在强酸性条件下,胺硼烷水解产生的析氢速率似乎比拟一级反应和使用文献中报道的速率常数所预测的要慢得多。这表明,在升高的酸度(5 mol L-1 HCl 或 HClO4)下,胺硼烷的水解分两步进行,产生第一批 H2 (0.67-1.15 mol) 比剩余的约 2 mol 快得多。这一证据表明一种与文献中所接受的胺硼烷水解机制不同的水解机制。在升高的酸度下通过胺硼烷还原无机 SeIV 观察到的 H2Se 的相对高效率可以归因于硼烷中间体的作用,最可能是胺硼烷阳离子,在相同反应条件下胺硼烷水解期间形成。
更新日期:2018-01-01
中文翻译:

胺硼烷在用于化学蒸汽发生的酸性介质中的水解行为和动力学
NaBH4 (THB) 和胺硼烷、NH3BH3 (AB)、叔丁基 NH2BH3 (TBAB)、Me2NHBH3 (DMAB) 在连续流动化学气相生成 H2Se 的 SeIV 水溶液中结合原子吸收光谱进行了研究。与 THB 相比,使用毫摩尔浓度的还原剂 (0.001-0.1 mol L-1) 在强酸性条件下(HCl、HClO4、H2SO4、HNO3、0.5- 5 mol L-1 H+)。CVG 系统的分析适用性是通过在 0.5 M H2SO4 中使用 0.01 mol L-1 TBAB 测定天然水样认证参考材料中的 SeIV 来测试的。为了解释胺硼烷在 THB 方面出乎意料的更高效率,AB 的水解动力学,TBAB 和 DMAB 在酸性介质中进行了研究,该介质通常用于化学蒸汽发生以测定痕量元素。使用实验室制造的恒温反应池,通过监测水解过程中产生的氢气速率来研究动力学。在 0.1、0.5、5 mol L-1 HCl 或 HClO4 反应介质和 0.1 mol L-1 半胱氨酸 +0.1 mol L-1 HCl 或 HClO4 缓冲液中测量 AB、TBAB 和 DMAB 的动力学,反应时间为 0 到 30分钟 在强酸性条件下,胺硼烷水解产生的析氢速率似乎比拟一级反应和使用文献中报道的速率常数所预测的要慢得多。这表明,在升高的酸度(5 mol L-1 HCl 或 HClO4)下,胺硼烷的水解分两步进行,产生第一批 H2 (0.67-1.15 mol) 比剩余的约 2 mol 快得多。这一证据表明一种与文献中所接受的胺硼烷水解机制不同的水解机制。在升高的酸度下通过胺硼烷还原无机 SeIV 观察到的 H2Se 的相对高效率可以归因于硼烷中间体的作用,最可能是胺硼烷阳离子,在相同反应条件下胺硼烷水解期间形成。



























 京公网安备 11010802027423号
京公网安备 11010802027423号