当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Exceptionally High Proton and Lithium Cation Gas-Phase Basicity of the Anti-Diabetic Drug Metformin
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2017-11-02 00:00:00 , DOI: 10.1021/acs.jpca.7b09338 Ewa D. Raczyńska 1 , Jean-François Gal 2 , Pierre-Charles Maria 2 , Piotr Michalec 1 , Marcin Zalewski 1
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2017-11-02 00:00:00 , DOI: 10.1021/acs.jpca.7b09338 Ewa D. Raczyńska 1 , Jean-François Gal 2 , Pierre-Charles Maria 2 , Piotr Michalec 1 , Marcin Zalewski 1
Affiliation
|
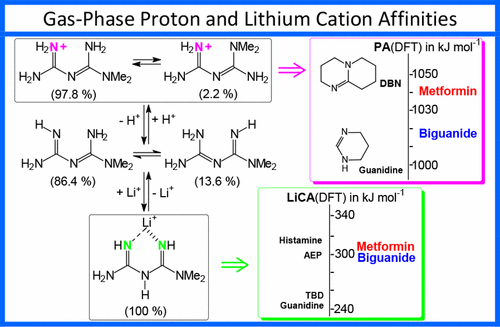
Substituted biguanides are known for their biological effect, and a few of them are used as drugs, the most prominent example being metformin (1,1-dimethylbiguanide, IUPAC name: N,N-dimethylimidodicarbonimidic diamide). Because of the presence of hydrogen atoms at the amino groups, biguanides exhibit a multiple tautomerism. This aspect of their structures was examined in detail for unsubstituted biguanide and metformin in the gas phase. At the density functional theory (DFT) level {essentially B3LYP/6-311+G(d,p)}, the most stable structures correspond to the conjugated, push–pull, system (NR2)(NH2)C═N–C(═NH)NH2 (R = H, CH3), further stabilized by an internal hydrogen bond. The structural and energetic aspects of protonation and lithium cation adduct formation of biguanide and metformin was examined at the same level of theory. The gas-phase protonation energetics reveal that the more stable tautomer is protonated at the terminal imino C═NH site, still with an internal hydrogen bond maintaining the structure of the neutral system. The calculated proton affinity and gas-phase basicity of the two molecules reach the domain of superbasicity. By contrast, the lithium cation prefers to bind the less stable, not fully conjugated, tautomer (NR2)C(═NH)–NH–C(═NH)NH2 of biguanides, in which the two C═NH groups are separated by NH. This less stable form of biguanides binds Li+ as a bidentate ligand, in agreement with what was reported in the literature for other metal cations in the solid phase. The quantitative assessment of resonance in biguanide, in metformin and in their protonated forms, using the HOMED and HOMA indices, reveals an increase in electron delocalization upon protonation. On the contrary, the most stable lithium cation adducts are less conjugated than the stable neutral biguanides, because the metal cation is better coordinated by the not-fully conjugated bidentate tautomer.
中文翻译:

抗糖尿病药物二甲双胍的超高质子和锂阳离子气相碱性
取代的双胍类化合物因其生物学作用而闻名,其中一些被用作药物,最突出的例子是二甲双胍(1,1-二甲基双胍,IUPAC名称:N,N-二甲基亚氨基二碳二亚氨基二酰胺)。由于氨基上存在氢原子,双胍类表现出多种互变异构现象。详细研究了气相结构中未取代的双胍和二甲双胍的结构。在密度泛函理论(DFT)级别{本质上为B3LYP / 6-311 + G(d,p)},最稳定的结构对应于共轭推挽系统(NR 2)(NH 2)C═N –C(═NH)NH 2(R = H,CH 3),通过内部氢键进一步稳定。在相同的理论水平上研究了双胍和二甲双胍的质子化和锂阳离子加合物形成的结构和能量方面。气相质子能学表明,更稳定的互变异构体在末端亚氨基C═NH位处被质子化,但仍具有保持中性系统结构的内部氢键。计算出的两个分子的质子亲和力和气相碱度达到了超碱性的范围。相比之下,锂阳离子更倾向于结合双胍的互变异构体(NR 2)C(═NH)–NH–C(═NH)NH 2由NH。双胍的这种较不稳定的形式结合了Li +作为双齿配体,与文献中关于固相中其他金属阳离子的报道一致。使用HOMED和HOMA指数对双胍,二甲双胍及其质子化形式的共振进行定量评估,结果表明质子化后电子离域作用增加。相反,最稳定的锂阳离子加合物比稳定的中性双胍的共轭少,因为金属阳离子与未完全共轭的双齿互变异构体能更好地配位。
更新日期:2017-11-03
中文翻译:

抗糖尿病药物二甲双胍的超高质子和锂阳离子气相碱性
取代的双胍类化合物因其生物学作用而闻名,其中一些被用作药物,最突出的例子是二甲双胍(1,1-二甲基双胍,IUPAC名称:N,N-二甲基亚氨基二碳二亚氨基二酰胺)。由于氨基上存在氢原子,双胍类表现出多种互变异构现象。详细研究了气相结构中未取代的双胍和二甲双胍的结构。在密度泛函理论(DFT)级别{本质上为B3LYP / 6-311 + G(d,p)},最稳定的结构对应于共轭推挽系统(NR 2)(NH 2)C═N –C(═NH)NH 2(R = H,CH 3),通过内部氢键进一步稳定。在相同的理论水平上研究了双胍和二甲双胍的质子化和锂阳离子加合物形成的结构和能量方面。气相质子能学表明,更稳定的互变异构体在末端亚氨基C═NH位处被质子化,但仍具有保持中性系统结构的内部氢键。计算出的两个分子的质子亲和力和气相碱度达到了超碱性的范围。相比之下,锂阳离子更倾向于结合双胍的互变异构体(NR 2)C(═NH)–NH–C(═NH)NH 2由NH。双胍的这种较不稳定的形式结合了Li +作为双齿配体,与文献中关于固相中其他金属阳离子的报道一致。使用HOMED和HOMA指数对双胍,二甲双胍及其质子化形式的共振进行定量评估,结果表明质子化后电子离域作用增加。相反,最稳定的锂阳离子加合物比稳定的中性双胍的共轭少,因为金属阳离子与未完全共轭的双齿互变异构体能更好地配位。


























 京公网安备 11010802027423号
京公网安备 11010802027423号