Catalysis Today ( IF 5.3 ) Pub Date : 2017-11-01 , DOI: 10.1016/j.cattod.2017.10.035 Yuepeng Pang , Tao Yuan , Junhe Yang , Mingxia Gao , Hongge Pan , Yongfeng Liu , Shiyou Zheng
|
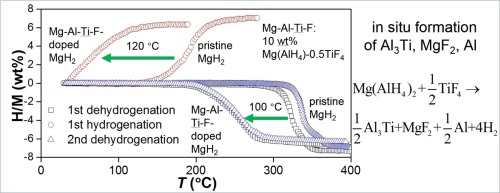
A mixture with the composition of MgH2–10 wt% (Mg(AlH4)2-0.5TiF4) (denoted as Mg-Al-Ti-F-doped MgH2) is designed and prepared by high-energy ball milling under the hydrogen pressure of 60 bar for 12 h. During the milling, a chemical reaction occurs between Mg(AlH4)2 and TiF4 to generate in situ Al3Ti, MgF2 and Al, which work together to induce a reduction of greater than 100 °C in the dehydrogenation temperature. At 275 °C, the Mg-Al-Ti-F-doped MgH2 rapidly releases 6.3 wt% H2 within 10 min in an isothermal experiment, while no appreciable hydrogen release is observed for the pristine MgH2 under identical conditions. The dehydrogenated Mg-Al-Ti-F-doped sample starts to take up hydrogen at room temperature with the 6.3 wt% amount of H2 at 150 °C, which is greatly superior to that of the pristine sample. A comprehensive kinetic analysis reveals that the superior functionality of the in situ formed Al3Ti, MgF2 and Al are mainly attributed to the following two factors: acting as nucleation centers and reducing the activation energy of growth.
中文翻译:

Al 3 Ti,MgF 2和Al的原位形成及其对MgH 2可逆储氢的优异协同作用
设计并通过在以下条件下进行高能球磨来制备具有MgH 2 -10 wt%(Mg(AlH 4)2 -0.5TiF 4)组成的混合物(表示为Mg-Al-Ti-F掺杂的MgH 2)。氢气压力为60 bar,持续12 h。在研磨过程中,Mg(AlH 4)2和TiF 4之间发生化学反应,原位生成Al 3 Ti,MgF 2和Al,它们共同作用导致脱氢温度降低超过100°C。在275°C下,掺杂Mg-Al-Ti-F的MgH 2快速释放6.3 wt%的H 2在等温实验中的10分钟内,没有发现在相同条件下原始MgH 2释放出明显的氢。掺有Mg-Al-Ti-F的脱氢样品在室温下开始吸收氢气,在150°C下的6.3 wt%H 2含量要大大优于原始样品。全面的动力学分析表明,原位形成的Al 3 Ti,MgF 2和Al的优异功能主要归因于以下两个因素:充当成核中心并降低生长的活化能。



























 京公网安备 11010802027423号
京公网安备 11010802027423号