当前位置:
X-MOL 学术
›
ACS Comb. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Stereoselective Synthesis of Benzo[e][1,4]oxazino[4,3-a][1,4]diazepine-6,12-diones with Two Diversity Positions
ACS Combinatorial Science ( IF 3.903 ) Pub Date : 2017-11-02 00:00:00 , DOI: 10.1021/acscombsci.7b00134 Petra Králová 1 , Michal Maloň 2 , Miroslav Soural 3
ACS Combinatorial Science ( IF 3.903 ) Pub Date : 2017-11-02 00:00:00 , DOI: 10.1021/acscombsci.7b00134 Petra Králová 1 , Michal Maloň 2 , Miroslav Soural 3
Affiliation
|
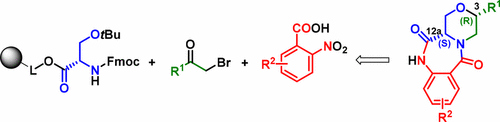
Herein, we report a stereoselective formation of tetrahydro-6H-benzo[e][1,4]oxazino[4,3-a][1,4]diazepine-6,12(11H)-diones. Their preparation consisted in solid-phase synthesis of linear intermediates starting from polymer-supported Ser(tBu)-OH. Using various 2-nitrobenzoic acids and bromoketones, the key intermediates were obtained in five steps and subjected to trifluoroacetic acid-mediated cleavage from the resin, followed by stereoselective reduction with triethylsilane. Subsequent catalytic hydrogenation of the nitro group and cyclization yielded the target compounds with full retention of the C12a stereocenter configuration.
中文翻译:

具有两个多样性位置的苯并[ e ] [1,4]恶嗪基[4,3- a ] [1,4]二氮杂-6,12-二酮的立体选择性合成
在此,我们报道了四氢-6 H-苯并[ e ] [1,4]恶嗪基[4,3- a ] [1,4]二氮杂-6,12 (11 H)-二酮的立体选择性形成。他们的制备包括从聚合物负载的Ser(t Bu)-OH开始的线性中间体的固相合成。使用各种2-硝基苯甲酸和溴代酮,可以通过五个步骤获得关键中间体,并从树脂进行三氟乙酸介导的裂解,然后用三乙基硅烷进行立体选择性还原。随后硝基的催化加氢和环化产生具有完全保留的C 12a立体中心构型的目标化合物。
更新日期:2017-11-02
中文翻译:

具有两个多样性位置的苯并[ e ] [1,4]恶嗪基[4,3- a ] [1,4]二氮杂-6,12-二酮的立体选择性合成
在此,我们报道了四氢-6 H-苯并[ e ] [1,4]恶嗪基[4,3- a ] [1,4]二氮杂-6,12 (11 H)-二酮的立体选择性形成。他们的制备包括从聚合物负载的Ser(t Bu)-OH开始的线性中间体的固相合成。使用各种2-硝基苯甲酸和溴代酮,可以通过五个步骤获得关键中间体,并从树脂进行三氟乙酸介导的裂解,然后用三乙基硅烷进行立体选择性还原。随后硝基的催化加氢和环化产生具有完全保留的C 12a立体中心构型的目标化合物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号