当前位置:
X-MOL 学术
›
Toxicol. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Evaluation of the toxicity of iron-ion irradiation in murine bone marrow dendritic cells via increasing the expression of indoleamine 2,3-dioxygenase 1
Toxicology Research ( IF 2.1 ) Pub Date : 2017-09-25 00:00:00 , DOI: 10.1039/c7tx00194k Yi Xie 1, 2, 3, 4, 5 , Jun-Fang Yan 1, 2, 3, 4, 5 , Jing-Yi Ma 4, 6, 7, 8 , Hong-Yan Li 1, 2, 3, 4, 5 , Yan-Cheng Ye 4, 9, 10 , Yan-Shan Zhang 4, 9, 10 , Hong Zhang 1, 2, 3, 4, 5
Toxicology Research ( IF 2.1 ) Pub Date : 2017-09-25 00:00:00 , DOI: 10.1039/c7tx00194k Yi Xie 1, 2, 3, 4, 5 , Jun-Fang Yan 1, 2, 3, 4, 5 , Jing-Yi Ma 4, 6, 7, 8 , Hong-Yan Li 1, 2, 3, 4, 5 , Yan-Cheng Ye 4, 9, 10 , Yan-Shan Zhang 4, 9, 10 , Hong Zhang 1, 2, 3, 4, 5
Affiliation
|
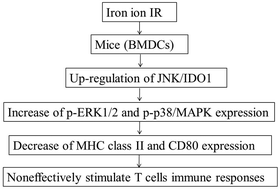
High linear energy transfer radiation is known to deposit higher energy in tissues and cause greater toxicity compared to low-LET irradiation. Local immunosuppression is frequently observed after irradiation (IR). Dendritic cells (DCs) play important roles in the initiation and maintenance of the immune response. The dysfunction of DCs contributes to tumor evasion and growth. However, molecular mechanisms underlying the establishment of immune tolerance induced by heavy ion IR through this DC population are poorly understood. Therefore, here we report our findings on the dysfunction of bone marrow-derived dendritic cells (BMDCs) induced by 1 Gy iron ion radiation and promotions of expressions of JNK1/2/3, indoleamine 2,3-dioxygenase 1 (IDO1), p-ERK1/2 and p38/MAPK; and decrease of IDO2, MHC class II, CD40, CD80 expressions and IFN-γ and TNF-α secretion after total-body IR in mice. JNK+IDO1+ BMDCs showed up-expression of p-ERK1/2 and p-p38/MAPK, reduced expression of MHC class II and CD80, and were not able to effectively stimulate allogeneic spleen T cells. The inhibition of IDO1 expressions could partly restore the function of BMDCs. In all, our study shows that elevated JNK and IDO1 expression induced by Fe ion IR could result in dysfunction of BMDCs via p-p38/MAPK and p-ERK1/2 signal pathway, and it may represent a new mechanism in radiation-induced immune tolerance.
中文翻译:

通过增加吲哚胺2,3-双加氧酶1的表达评估铁离子辐照对小鼠骨髓树突状细胞的毒性
与低LET辐射相比,已知高线性能量转移辐射会在组织中沉积更高的能量并引起更大的毒性。辐射(IR)后经常观察到局部免疫抑制。树突状细胞(DC)在免疫反应的启动和维持中起重要作用。DC的功能障碍导致肿瘤逃避和生长。但是,通过重离子IR通过该DC种群建立免疫耐受的分子机制了解甚少。因此,在这里我们报告了我们的发现:1 Gy铁离子辐射诱导的骨髓源性树突状细胞(BMDCs)的功能障碍以及JNK1 / 2/3,吲哚胺2,3-二加氧酶1(IDO1),p的表达增强-ERK1 / 2和p38 / MAPK; 和减少IDO2,MHC II类,CD40,小鼠全身IR后CD80的表达以及IFN-γ和TNF-α的分泌。JNK+ IDO1 + BMDCs显示p-ERK1 / 2和p-p38 / MAPK的上调表达,降低MHC II类和CD80的表达,并且不能有效刺激同种异体脾T细胞。抑制IDO1表达可以部分恢复BMDC的功能。总之,我们的研究表明,铁离子IR诱导的JNK和IDO1表达升高可能通过p-p38 / MAPK和p-ERK1 / 2信号通路导致BMDC的功能障碍,这可能代表了辐射诱导免疫的新机制。宽容。
更新日期:2017-10-30
中文翻译:

通过增加吲哚胺2,3-双加氧酶1的表达评估铁离子辐照对小鼠骨髓树突状细胞的毒性
与低LET辐射相比,已知高线性能量转移辐射会在组织中沉积更高的能量并引起更大的毒性。辐射(IR)后经常观察到局部免疫抑制。树突状细胞(DC)在免疫反应的启动和维持中起重要作用。DC的功能障碍导致肿瘤逃避和生长。但是,通过重离子IR通过该DC种群建立免疫耐受的分子机制了解甚少。因此,在这里我们报告了我们的发现:1 Gy铁离子辐射诱导的骨髓源性树突状细胞(BMDCs)的功能障碍以及JNK1 / 2/3,吲哚胺2,3-二加氧酶1(IDO1),p的表达增强-ERK1 / 2和p38 / MAPK; 和减少IDO2,MHC II类,CD40,小鼠全身IR后CD80的表达以及IFN-γ和TNF-α的分泌。JNK+ IDO1 + BMDCs显示p-ERK1 / 2和p-p38 / MAPK的上调表达,降低MHC II类和CD80的表达,并且不能有效刺激同种异体脾T细胞。抑制IDO1表达可以部分恢复BMDC的功能。总之,我们的研究表明,铁离子IR诱导的JNK和IDO1表达升高可能通过p-p38 / MAPK和p-ERK1 / 2信号通路导致BMDC的功能障碍,这可能代表了辐射诱导免疫的新机制。宽容。

























 京公网安备 11010802027423号
京公网安备 11010802027423号