Bioorganic & Medicinal Chemistry Letters ( IF 2.7 ) Pub Date : 2017-10-28 , DOI: 10.1016/j.bmcl.2017.10.071 Yasodakrishna Sajja , Sowmya Vanguru , Hanmanth Reddy Vulupala , Rajashaker Bantu , Perumal Yogeswari , Dharmarajan Sriram , Lingaiah Nagarapu
|
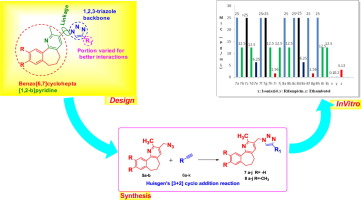
A series of novel benzo[6,7]cyclohepta[1,2-b]pyridine-1,2,3-triazole hybrids (7a–j & 8a–j) have been designed and synthesized in excellent yields by Huisgen’s [3+2] cyclo addition reaction of 3-(azidomethyl)-2-methyl-6,7-dihydro-5H-benzo[6,7]cyclohepta[1,2-b]pyridine (5) with various alkynes 6 in presence of copper sulphate and sodium ascorbate and their structures were confirmed by IR, 1H NMR, 13C NMR and HRMS. The newly synthesized compounds 7a–j & 8a–j were evaluated for their in vitro anti-mycobacterial activity against Mycobacterium tuberculosis H37Rv (ATCC 27294). Among the compounds tested, the compounds 7i and 8g displayed most potent activity with MIC value of 1.56 µg/mL with low cytotoxicity.
中文翻译:

苯并[6,7]环庚[1,2 - b ]吡啶-1,2,3-三唑衍生物的设计,合成及体外抗结核活性
Huisgen [3+]设计并合成了一系列新颖的苯并[6,7]环庚[1,2 - b ]吡啶-1,2,3-三唑杂种(7a–j和8a–j)。 2]的3-(叠氮基甲基)环加成反应-2-甲基-6,7-二氢-5- ħ -苯并[6,7]环庚并[1,2- b ]吡啶(5)具有在存在各种炔6硫酸铜和抗坏血酸钠及其结构通过IR,1 H NMR,13 C NMR和HRMS确认。对新合成的化合物7a–j和8a–j进行了体外评估抗结核分枝杆菌H37Rv(ATCC 27294)的抗分枝杆菌活性。在测试的化合物中,化合物7i和8g表现出最强的活性,MIC值为1.56 µg / mL,细胞毒性较低。



























 京公网安备 11010802027423号
京公网安备 11010802027423号