Bioorganic & Medicinal Chemistry ( IF 3.5 ) Pub Date : 2017-10-26 , DOI: 10.1016/j.bmc.2017.10.029 Akiko Asano , Shohei Numata , Takeshi Yamada , Katsuhiko Minoura , Mitsunobu Doi
|
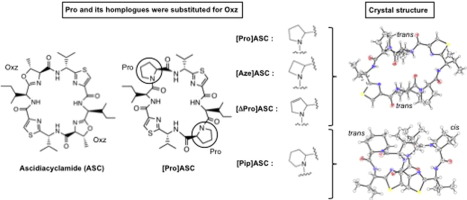
Ascidiacyclamide [ASC, cyclo(-Ile-oxazoline-d-Val-thiazole-)2] is a cyclic octapeptide isolated from tunicates. We designed ASC analogues [cyclo(-Ile-Xxx-d-Val-thiazole-)2] in which Pro or a homologue was substituted for oxazoline: [Pro]ASC (Xxx: proline), [Aze]ASC (Xxx: (S)-Azetidine-2-carboxylic acid), [Pip]ASC (Xxx: (S)-Piperidine-2-carboxylic acid) and [ΔPro]ASC (Xxx: (S)-3-pyrroline-2-carboxylic acid) to explore their potential to serve as substitutes for the oxazoline ring. The conformations of these analogues were examined using X-ray diffraction, 1H NMR and CD spectroscopy. In both the crystal and solution states, the conformations of [Pro]ASC, [Aze]ASC and [ΔPro]ASC were novel square structures having two trans imide bonds and stabilized by two intramolecular hydrogen bonds. The crystal structure of [Pip]ASC was a folded conformation with cis and trans imide bonds. Three isomers (cc, ct and tt) were present in a solution of [Pip]ASC. From crystal structures, the degree of puckering in the side chains of Pro and its homologues was estimated to be in the order Pip > Pro > Aze > ΔPro. [Pro]ASC and [Pip]ASC showed strong cytotoxicity, but [Aze]ASC and [ΔPro]ASC showed no cytotoxicity. Among the four analogues, there is consistency between the prolyl ring puckering and cytotoxicity, but not between the peptide backbone structure and cytotoxicity.
中文翻译:

环二酰胺类似物与环状α-氨基酸而不是恶唑啉残基的构象性质
顺式二环酰胺[ASC,环(-Ile-恶唑啉-d - Val-噻唑-)2 ]是从被膜中分离出的环状八肽。我们设计了ASC类似物[cyclo(-Ile-Xxx- d -Val-噻唑-)2 ],其中Pro或同系物取代了恶唑啉:[Pro] ASC(Xxx:脯氨酸),[Aze] ASC(Xxx:(S)-氮杂环丁烷-2-羧酸),[Pip] ASC(Xxx:(S)-哌啶-2-羧酸)和[ΔPro] ASC(Xxx:(S)-3-吡咯啉-2-羧酸)探索它们作为恶唑啉环替代物的潜力。使用X射线衍射检查了这些类似物的构象,11 H NMR和CD光谱。在晶体状态和溶液状态下,[Pro] ASC,[Aze] ASC和[ΔPro] ASC的构型都是具有两个反酰亚胺键并被两个分子内氢键稳定的新型方形结构。[Pip] ASC的晶体结构为顺式和反式折叠构型酰亚胺键。[Pip] ASC溶液中存在三种异构体(cc,ct和tt)。从晶体结构来看,Pro及其同系物的侧链中的褶皱程度估计为Pip> Pro> Aze>ΔPro。[Pro] ASC和[Pip] ASC显示出很强的细胞毒性,但是[Aze] ASC和[ΔPro] ASC没有显示出细胞毒性。在这四个类似物中,脯氨酰环起皱和细胞毒性之间具有一致性,而肽主链结构和细胞毒性之间没有一致性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号