Catalysis Today ( IF 5.3 ) Pub Date : 2017-10-26 , DOI: 10.1016/j.cattod.2017.10.037 J.C. García-Martínez , H.A. González Uribe , M.M. González-Brambila , J.A. Colín-Luna , Y.E. Escobedo-García , A. López-Gaona , L. Alvarado-Perea
|
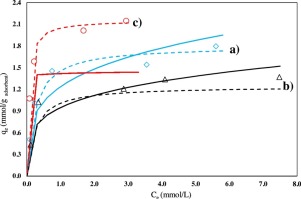
The adsorption of quinoline (Q) and dibenzothiophene (DBT)—model compounds for nitrogen and sulfur in diesel fuels—over mesoporous SBA-15, SBA-16, and MCM-41 was studied. The Langmuir model was suitable for describing the adsorption of nitrogen-containing compounds from a simulated diesel fuel. A pseudo-second-order kinetic model better fitted the Q adsorption data than a first-order rate model when describing the adsorption rates on all materials. Comparison of the adsorption of Q and DBT confirmed that the nitrogen compound was selectively removed, and MCM-41 was found to have better adsorption characteristics than SBA-15 and SBA-16. DBT was not adsorbed in any experiment. The adsorbents were characterized using N2-physisorption, powder X-ray diffraction, and high-resolution transmission electron microscopy to describe the morphologies of the adsorbents. The characterization results revealed that the specific area and the structure of the adsorbent are key parameters required to explain the adsorption process.
中文翻译:

使用二氧化硅基介孔材料选择性吸附氮化合物作为深度加氢脱硫的预处理
研究了介孔SBA-15,SBA-16和MCM-41对喹啉(Q)和二苯并噻吩(DBT)(柴油中氮和硫的模型化合物)的吸附。Langmuir模型适用于描述模拟柴油燃料中含氮化合物的吸附。当描述所有材料的吸附速率时,伪二级动力学模型比一级速率模型更好地拟合了Q吸附数据。比较Q和DBT的吸附,证实了氮化合物被选择性地除去,并且发现MCM-41具有比SBA-15和SBA-16更好的吸附特性。在任何实验中都没有吸附DBT。用N 2表征吸附剂-物理吸附,粉末X射线衍射和高分辨率透射电子显微镜来描述吸附剂的形态。表征结果表明,吸附剂的比表面积和结构是解释吸附过程所需的关键参数。



























 京公网安备 11010802027423号
京公网安备 11010802027423号